CHARLOTTE, N.C.—Patients being treated with vedolizumab or ustekinumab for inflammatory bowel disease who are pregnant or are planning a pregnancy can be assured that it is safe for them to continue these agents throughout pregnancy, according to new research.
Data from the Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes (PIANO) registry had demonstrated the safety of biologics and thiopurines in pregnant patients, but until recently there were only small numbers on use of vedolizumab (Entyvio, Takeda) and ustekinumab (Stelara, Janssen), said investigator Rishika Chugh, MD, who presented the research at the 2022 annual meeting of the American College of Gastroenterology.
Previously, the mechanism of action of both drugs caused apprehension about use in pregnant patients. Vedolizumab prevents leukocyte movement to the gut, but the receptor it binds also “plays an integral role in placenta development,” said Dr. Chugh, an assistant professor of medicine at the University of California, San Francisco. “This raises the concern that vedolizumab exposure could increase the incidence of placental disorders.” Ustekinumab targets interleukin (IL)-12, which functions in uterine angiogenesis, and IL-13, which regulates the function of human decidual immune cells in early pregnancy. “Both elevated and absent levels of these cytokines are associated with spontaneous abortion,” Dr. Chugh said.
To investigate the safety of the two monoclonal antibodies, she and her co-investigators administered questionnaires to women participating in the PIANO registry at the time of study intake, throughout their pregnancy and after delivery (abstract 43/S719). The participants included 43 ustekinumab-exposed pregnancies, 62 vedolizumab-exposed pregnancies, 430 unexposed controls, 700 patients taking tumor necrosis factor (TNF) inhibitors, 226 patients taking immunomodulators and 179 taking combination therapy.
At the time of the study analysis, 1,669 patients had completed pregnancies, with 1,610 live births. Maternal age, body mass index and number of pregnancies were similar among all groups, but IBD duration was significantly longer in the ustekinumab cohort, at a median of 14 years compared with 9.6 years in the vedolizumab cohort. In addition, more patients were diagnosed with Crohn’s disease (83%) than ulcerative colitis in the ustekinumab group compared with the vedolizumab group (48%).
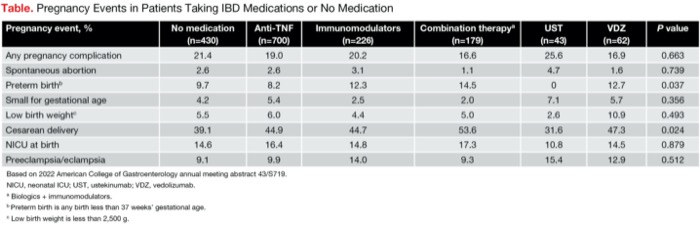
Adverse pregnancy events, including overall pregnancy complications, spontaneous abortion and number of infants born small for gestational age, were similar among all groups (Table). “However, there were significantly fewer preterm births in the ustekinumab-exposed cohort—no preterm births out of the 42 patients with available data,” Dr. Chugh said.
| Table. Pregnancy Events in Patients Taking IBD Medications or No Medication | |||||||
| Pregnancy event, % | No medication (n=430) | Anti-TNF (n=700) | Immuno- modulators (n=226) | Combination therapya (n=179) | UST (n=43) | VDZ (n=62) | P value |
|---|---|---|---|---|---|---|---|
| Any pregnancy complication | 21.4 | 19.0 | 20.2 | 16.6 | 25.6 | 16.9 | 0.663 |
| Spontaneous abortion | 2.6 | 2.6 | 3.1 | 1.1 | 4.7 | 1.6 | 0.739 |
| Preterm birthb | 9.7 | 8.2 | 12.3 | 14.5 | 0 | 12.7 | 0.037 |
| Small for gestational age | 4.2 | 5.4 | 2.5 | 2.0 | 7.1 | 5.7 | 0.356 |
| Low birth weightc | 5.5 | 6.0 | 4.4 | 5.0 | 2.6 | 10.9 | 0.493 |
| Cesarean delivery | 39.1 | 44.9 | 44.7 | 53.6 | 31.6 | 47.3 | 0.024 |
| NICU at birth | 14.6 | 16.4 | 14.8 | 17.3 | 10.8 | 14.5 | 0.879 |
| Preeclampsia/ eclampsia | 9.1 | 9.9 | 14.0 | 9.3 | 15.4 | 12.9 | 0.512 |
| Based on 2022 American College of Gastroenterology annual meeting abstract 43/S719. NICU, neonatal ICU; UST, ustekinumab; VDZ, vedolizumab. a Biologics + immunomodulators. b Preterm birth is any birth less than 37 weeks’ gestational age. c Low birth weight is less than 2,500 g. | |||||||
There were no differences among the multiple cohorts in low birth weight, intrauterine growth restriction, neonatal ICU admission at birth and congenital malformations. The ustekinumab-treated patients had a significantly lower rate of cesarean delivery than the vedolizumab cohort (31.6% and 47.3%, respectively).
The findings showed no increased signal for placental disorders, such as placental abruption, preeclampsia and eclampsia, placenta previa, hemorrhage, and postpartum hemorrhage, associated with either agent.
“At this time, continuation of ustekinumab and vedolizumab during pregnancy is recommended,” Dr. Chugh said.
Goals for Remission Throughout Pregnancy
The findings are welcome news to David P. Hudesman, MD, the co-director of the Inflammatory Bowel Disease Center at NYU Langone Health, in New York City.
“This is one of the most common questions we get from female patients of childbearing age, whether they’re thinking about getting pregnant in the near future or actively trying to get pregnant: Is it safe for them to continue their medications?” Dr. Hudesman said.
His patients’ first major concern is whether their IBD treatment will have a negative effect on the baby or the pregnancy, and their second worry is what will happen to management of their disease if they alter their medication.
“We’ve had some smaller studies in the past and preliminary data from the PIANO registry showing the safety of our biologics throughout pregnancy and during breastfeeding. But in talking to patients, we still hear some nervousness or concern, so it’s really important to have a large amount of prospective data to go over with patients and answer some of their questions,” Dr. Hudesman said.
He noted that since the biggest risk in pregnancy is active inflammation, keeping IBD under control is of utmost concern. “The most important thing for our patients is to go into pregnancy while in remission and stay on therapy so they can remain in remission throughout the pregnancy,” Dr. Hudesman said. “This study showed that both agents are safe to continue. That should be our standard and what we recommend to all patients.”
—Monica J. Smith
Dr. Chugh reported no relevant financial disclosures. Dr. Hudesman reported financial relationships with Janssen and Takeda.
This article is from the March 2023 print issue.