mt-sDNA
FDA Approves Next-Gen mt-sDNA test, Cologuard Plus
The FDA has approved the next-generation of the multitarget stool DNA test Cologuard (Exact Sciences).
OCTOBER 9, 2024

Colonic Lesion Detection After Stool Test, PPI Use and AI Bowel Prep Evaluation
In this month’s column, I cover three topics: detection rates after stool tests, inappropriate use of PPIs, ...
JULY 17, 2024

Blood-Based CRC Screening Tests: AGA Reacts
Data emerging on blood-based testing for colorectal cancer have prompted questions from gastroenterologists and ...
MAY 23, 2024
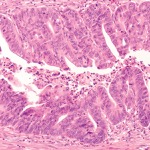
First-Line Options for CRC Screening: Stool-Based Screening Versus Colonoscopy
For colorectal cancer prevention, should stool-based testing be a first-line option rather than just an alternative ...
APRIL 29, 2024

Blood-Based Tests Will Be Game Changers For Cancer Screening
To meet the increased CRC screening demands, clinicians need to use all available modalities—including stool- ...
APRIL 1, 2024
Zero Coinsurance for Screening Colonoscopy After Positive Stool Test Could Up Patient Life-Years
Cutting out coinsurance for patients undergoing colonoscopy after a positive stool test could improve patient ...
JANUARY 11, 2024

Expert Picks From ACG 2023, Part 1
In this installment of “Expert Picks,” Brooks D. Cash, MD, discusses four of his favorite abstracts ...
DECEMBER 26, 2023
New Research, Guidelines and More That Changed Gastroenterology in 2023
The gastroenterology field was full of new developments in 2023.
DECEMBER 21, 2023

Multitarget Stool DNA Has Similar Accuracy in Patients After Bariatric Sx
mt-sDNA testing is accurate in predicting advanced colorectal neoplasia in patients who have undergone bariatric ...
NOVEMBER 7, 2023

Noninvasive CRC Screening Tests Continue to Demonstrate Promise
Colonoscopy for colorectal cancer screening may not be a thing of the past yet, but two noninvasive screening tests ...
OCTOBER 11, 2023

