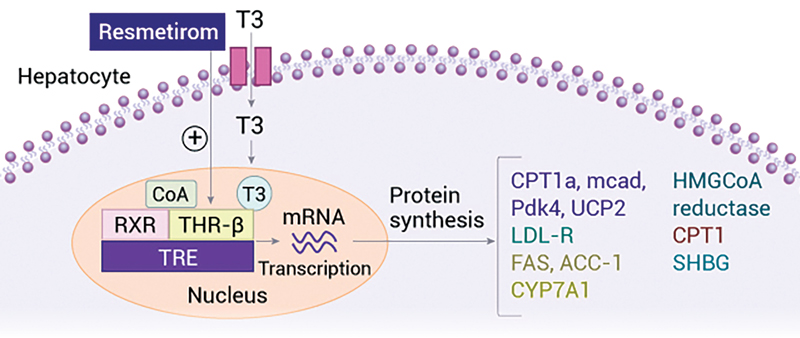
Patients with metabolic dysfunction–associated steatohepatitis and F2 or F3 fibrosis treated with resmetirom had improvements in both fibrosis and MASH over 52 weeks of treatment, according to several substudies from the phase 3 MAESTRO-NASH trial presented at EASL Congress 2024.
The ongoing randomized, double-blind, placebo-controlled trial involves 966 patients randomly assigned to receive 80 or 100 mg of resmetirom (Rezdiffra, Madrigal) or placebo daily for 54 months. At week 52, the researchers evaluated the resolution of MASH with no worsening of fibrosis or a reduction in fibrosis stage with no worsening of MASH, examining variables such as body mass index, metabolic alcohol-associated liver disease (MetALD), quality of life and disease complications.
Ashwani K. Singal, MD, MS, a professor of medicine at the University of Louisville, in Kentucky, and the chair of the American Gastroenterological Association Institute Council Liver & Biliary Section, said he found the results “very encouraging.” In an interview with GEN Priority Report, Dr. Singal said, “The important message is that the patients are tolerating it. They are stable or improving, so providers can encourage patients to continue the treatment to improve long-term outcomes. When the study is completed in 2026, I won’t be surprised [if] everything comes in favor of the medication and there is full approval of the drug.”
Benefits With Higher BMI
A subanalysis of MAESTRO-NASH presented at EASL found that for patients with higher baseline body weight and BMI, resmetirom 100 mg was more effective than 80 mg in achieving the dual primary end points (abstract OS-117). However, there was no significant difference in efficacy between the two doses for patients with a lower BMI.
Patients weighing more than 100 kg had a 30% proton density fat fraction (PDFF) response of 56.7% when taking 80 mg, compared with 72.5% with 100 mg. They also saw more improvement in fibrosis and MASH resolution with higher dosages (fibrosis: 80 mg, 22.5%; 100 mg, 32.5%; MASH resolution: 80 mg, 27%; 100 mg, 32.5%).
However, patients on both doses of resmetirom with a BMI less than 35 kg/m2 showed greater improvement in fibrosis and a lower rate of worsening than those with a BMI of 35 kg/m2 or higher.
Similar Response in MetALD
In another subanalysis, response rates for resmetirom were similar in patients with suspected MetALD and those without it (abstract SAT-263).
Of the 782 patients who had both a baseline and week 52 liver biopsy, 75 (9.6%) fell into MetALD category based on a carbohydrate deficient transferrin more than 2.5% and/or a phosphatidylethanol greater than 20 ng/mL, and 707 did not.
Response rates to resmetirom were similar in the two groups. MASH resolution was similar in the patients with and without MetALD (80 mg, 29% vs. 29.9%; 100 mg, 35% vs. 36%, respectively), as was fibrosis improvement (80 mg, 29% vs. 35%; 100 mg, 33.3% vs. 30%). Higher percentages of patients with MetALD achieved a 30% or higher reduction in MRI-PDFF (80 mg, 59.2% vs. 88%; 100 mg, 71.2% vs. 81%).
Enhancing Quality of Life
Patients with MASH who showed fibrosis improvement or MASH resolution when treated with resmetirom also experienced significant benefit across various aspects of health-related quality of life (HRQOL) after completing 52 weeks of treatment, according to another subanalysis (abstract FRI-202).
The study aimed to evaluate HRQOL in noncirrhotic MASH patients with confirmed or suspected fibrosis who received treatment with resmetirom, using the Chronic Liver Disease Questionnaire–NASH (score range, 1-7) and Liver Disease Quality of Life (score range, 0-100).
The pooled group of patients receiving 80- or 100-mg dosages demonstrated significant improvements in multiple domains of HRQOL scores, including Worry (+0.46; minimally clinically important difference [MCID], 41%), Role Emotional (+3.0; MCID, 28%), Health Distress (+8.1; MCID, 38%), Stigma (+3.5; MCID, 39%), and total Liver Disease Quality of Life (+2.2; MCID, 35%). Patients who responded to resmetirom showed similar improvements in HRQOL scores, whereas the placebo group and nonresponders did not show improvements.
—Norah Chinn
The MAESTRO-NASH study is supported by Madrigal. Dr. Singal reported no relevant financial disclosures. He is a member of the Gastroenterology & Endoscopy News editorial board.
This article is from the December 2024 print issue.