The American Gastroenterological Association has issued an updated clinical practice guideline on prevention and management of hepatitis B virus reactivation in patients on immunosuppressive medication.
Recommendations in the new guideline are based on patient risk status and range from antiviral prophylaxis for those at high and moderate risk to careful monitoring for low-risk patients (Gastroenterology 2025;168[2]: 267-284). Risk categorization is driven by the baseline risk of HBV reactivation, and guideline authors recommend screening all at-risk individuals for serological markers alone, followed by viral load testing in patients who test positive.
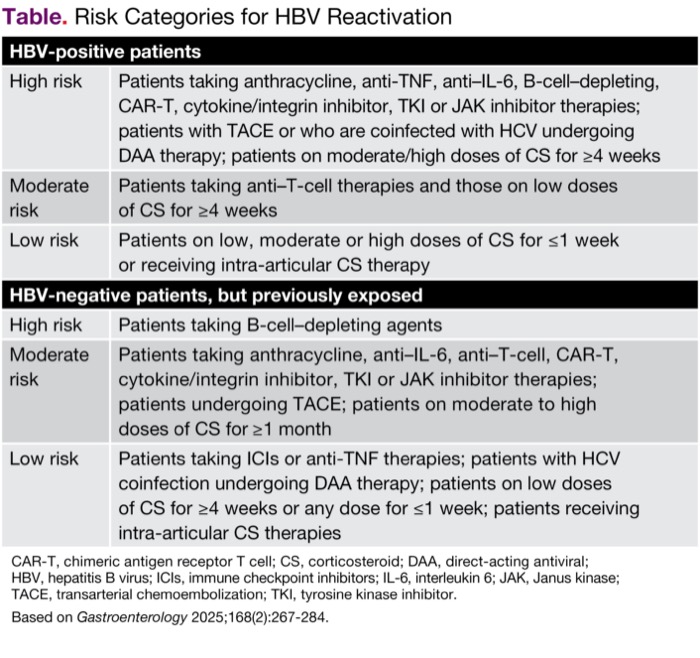
Patients at risk for HBV reactivation include patients who are HB surface antigen (HBsAg)-positive and those with a resolved HBV infection (HBsAg-negative, HBV DNA–negative and anti–HBc-positive); the former group is at a relatively higher risk of reactivation than the latter (Table).
| Table. Risk Categories for HBV Reactivation | |
| HBV-positive patients | |
|---|---|
| High risk | Patients taking anthracycline, anti-TNF, anti–IL-6, B-cell–depleting, CAR-T, cytokine/integrin inhibitor, TKI or JAK inhibitor therapies; patients with TACE or who are coinfected with HCV undergoing DAA therapy; patients on moderate/high doses of CS for =4 weeks |
| Moderate risk | Patients taking anti–T-cell therapies and those on low doses of CS for =4 weeks |
| Low risk | Patients on low, moderate or high doses of CS for =1 week or receiving intra-articular CS therapy |
| HBV-negative patients, but previously exposed | |
| High risk | Patients taking B-cell–depleting agents |
| Moderate risk | Patients taking anthracycline, anti–IL-6, anti–T-cell, CAR-T, cytokine/integrin inhibitor, TKI or JAK inhibitor therapies; patients undergoing TACE; patients on moderate to high doses of CS for =1 month |
| Low risk | Patients taking ICIs or anti-TNF therapies; patients with HCV coinfection undergoing DAA therapy; patients on low doses of CS for =4 weeks or any dose for =1 week; patients receiving intra-articular CS therapies |
| CAR-T, chimeric antigen receptor T cell; CS, corticosteroid; DAA, direct-acting antiviral; HBV, hepatitis B virus; ICIs, immune checkpoint inhibitors; IL-6, interleukin 6; JAK, Janus kinase; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor. Based on Gastroenterology 2025;168(2):267-284. | |
Noting that myriad immune modulating drugs that can increase a patient’s risk for HBV reactivation have been approved for clinical use since the previous guideline on HBV reactivation prevention was published in 2014, the panel sought to marshal available data on these therapies to provide clear guidance for clinicians. Examples of these drugs include anti-interleukin (IL) therapies, Janus kinase inhibitors and cytokine/integrin inhibitors used to treat gastrointestinal and rheumatologic diseases and dermatologic conditions, as well as immune checkpoint inhibitors, chimeric antigen receptor T-cell (CAR-T) therapies and tyrosine kinase inhibitors to treat cancer. Guideline authors also curated updated evidence on established drugs such as anti–tumor necrosis factor (TNF) drugs.
The guideline panel strongly recommended that people at high risk for HBV reactivation take antiviral prophylaxis with a high barrier to resistance before starting medications that have the potential to reactivate the virus and continue until six months after therapy discontinuation.
They also recommended prophylaxis over monitoring in patients at moderate risk but noted that those who are reluctant to antiviral therapy “may reasonably select active monitoring,” which should include alanine aminotransferase and HBV assessment every one to three months. They suggested that monitoring alone is sufficient for low-risk patients.
“We did an empirical evaluation of the baseline risk of HBV reactivation, as there are some drugs that are for sure high risk (>10% risk for reactivation), but there are other scenarios where we’re not going to worry about reactivation, such as if someone’s getting a steroid injection in their knee,” guideline author Faisal S. Ali, MD, told Gastroenterology & Endoscopy News. The updated body of evidence on older medications such as anti–TNF drugs also warranted recategorization of their risk class, he added.
“There is not enough evidence yet to make a determination about risk associated with some newer drugs, such as the integrin inhibitors for IBD, for example, vedolizumab,” he said. “In such cases, a recommendation has been made based on biological plausibility.” Dr. Ali noted that as data is released on newer therapies, another update is expected in about five years.
—Katie Prince