Medical Director
Digestive Health Center
MetroHealth
Cleveland, Ohio
In this new column, I will focus on all things upper GI, from motility to Barrett’s esophagus and beyond, highlighting the latest research and updates on diagnostic and treatment modalities and how they affect clinical practice. In this month’s column, I highlight five studies presented at ACG 2024.
The first study, which I was a part of, is a post hoc analysis of on-demand treatment in patients with non-erosive reflux disease (NERD) using vonoprazan (Voquezna, Phathom), the first potassium-competitive acid blocker approved in the United States. In the original study, patients documented their symptomatic responses to vonoprazan every 30 minutes until resolution was achieved. This allowed the investigators, for the first time, to report on the “patient journey” after drug administration, demonstrating that patients experienced improvements in symptom severity before complete symptom resolution.
The next two studies used TriNetX, a de-identified global health research database encompassing more than 300 million patients. The first study evaluated the odds of all-cause mortality and the likelihood of initiating proton pump inhibitors in patients with gastroesophageal reflux disease (GERD) who were started on glucagon-like peptide-1 receptor agonist (GLP-1) treatment. The second study builds on recent literature suggesting that patients with Barrett’s esophagus are being diagnosed at younger ages. The investigators found that more patients younger than 50 years of age are being diagnosed with BE, and they also identified risk factors for BE in this younger population.
The fourth study focused on an area of great interest: the discordance between esophageal manometry, which evaluates primary peristalsis, and Endoflip (Medtronic) panometry, which assesses secondary peristalsis, in the same patients.
The final study examined the overutilization of sucralfate for upper gastrointestinal disorders, highlighting its growing expense despite limited therapeutic efficacy.
Heartburn Frequency and Symptom Improvement With On-Demand Vonoprazan for Non-Erosive Reflux Disease
ACG 2024 Presidential Plenary Session 1, Abstract 4
Investigators assessed the efficacy and safety of on-demand use of vonoprazan in patients with NERD who had symptom control after daily use of the drug. They noted that vonoprazan is thought to be a good candidate for on-demand treatment because of its rapid onset and durable acid suppression, as well as the fact that it is not food dependent.
Patients enrolled in a trial (ClinicalTrials.gov Identifier: NCT04799158) were eligible for the post hoc on-demand analysis if they were at least 80% adherent to four weeks of daily vonoprazan (20 mg), completed electronic diary entries and reported no heartburn during the fourth week of the study.
These patients (n=207) were randomized to six weeks of 10, 20 or 40 mg of vonoprazan or placebo, to be taken when they were experiencing heartburn, up to once every 24 hours. In an electronic diary, patients reported the severity of their heartburn when they took the treatment and every 30 minutes thereafter until three hours after treatment. Severity was reported as none, mild, moderate, severe or very severe.
Before they started daily vonoprazan, patients had an average of 16.1% of heartburn-free days. During the four weeks of daily vonoprazan, heartburn-free days increased to an average of 82.9% of days. During the on-demand treatment period, mean heartburn-free days stayed relatively high in all four treatment groups, ranging from 71.2% to 75%, overall, and were consistent across the six-week period.
Within the first hour of treatment, patients taking any vonoprazan dose were more likely than those taking placebo to have heartburn severity improvement of at least one grade (10 mg: 75.5%, P<0.0001; 20 mg: 69.1%, P=0.0010; 40 mg: 75.5%, P<0.0001; placebo: 57.0%). By two hours after treatment, more than 90% of patients in each vonoprazan group had symptom improvement of at least one grade.
GLP-1 Agonists and Outcomes In GERD Patients
ACG 2024 Plenary Session 3A, Abstract 37
A multicenter team of researchers conducted a retrospective cohort study to assess outcomes in patients with GERD who initiate GLP-1s. The investigators noted that while there is some evidence of GLP-1 use being associated with incidence of GERD, there are less data on outcomes of patients who already have GERD and begin treatment with GLP-1s.
Using data from the TriNetX database, the researchers identified adults with GERD who had no history of complications and were taking medications for GERD other than GLP-1s. The researchers identified 2,760 such patients who had started a GLP-1 between 2010 and 2023 and 270,869 patients who had not taken a GLP-1 during that time. Using propensity score matching based on age, demographic factors and relevant comorbidities, the investigators matched 1,101 GERD patients taking GLP-1s in a 1:1 fashion to those not taking a GLP-1.
The investigators, led by Luis Nieto, MD, a digestive diseases fellow at Emory University School of Medicine, in Atlanta, compared the odds of all-cause mortality in those taking GLP-1s and those who had not taken them, as well as the odds of GERD complications, esophageal cancer, procedure requirements and PPI use.
The patients with GERD who took GLP-1s had lower odds of all-cause mortality compared with the those who had not taken a GLP-1 (odds ratio [OR], 0.18; 95% CI, 0.13-0.25). In addition, those taking GLP-1s had lower odds of initiating PPIs (OR, 0.78; 95% CI, 0.62-0.98). There were no differences in the odds of incidence of BE, esophagitis, esophageal stricture, esophageal cancer or asthma; esophagogastroduodenoscopy or esophageal manometry requirements; or fundoplication.
Increasing Incidence of Young-Onset Barrett’s Esophagus
ACG 2024 Plenary Session 3A, Abstract 39
Based on the increasing incidence of esophageal adenocarcinoma (EAC) among patients younger than 50 years of age, investigators, led by Anila Vasireddy, MD, an internal medicine resident at the University of Pennsylvania Health System, in Philadelphia, investigated trends in the epidemiology of BE.
The investigators used data from the TriNetX database to identify patients with incident BE, including non-dysplastic BE, dysplastic BE and EAC, who had a negative EGD before BE diagnosis, and non-BE controls. Patients were identified using ICD 9/10 and CPT codes.
Among nearly 2.7 million patients included, 3.8% were incident BE cases (n=102,598). Nearly 20% of incident BE cases were in those younger than 50 years of age, and the investigators found the incidence of young-onset BE increased by 2% annually over the study period (2014-2023). Most of these cases of young-onset BE were non-dysplastic BE at diagnosis (94%), but 3.6% had EAC.
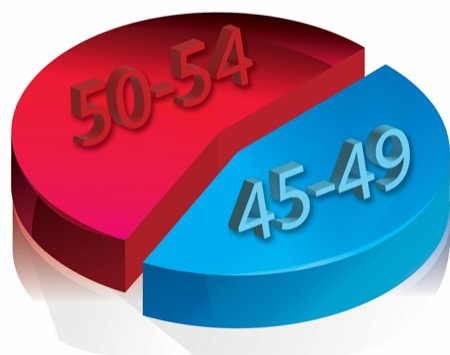
Of note, the incidence of BE in those 45 to 49 years of age, who do not generally fall under current screening guidelines, was comparable to the incidence in those between 50 and 54, who are currently included in the guidelines.
Among those younger than 50, factors significantly associated with incidence of BE included male sex (OR, 1.89; 95% CI, 1.80-1.99), white race (OR, 2.23; 95% CI, 2.08-2.40), obesity (OR, 1.82; 95% CI, 1.73-1.91), having GERD symptoms (OR, 1.22; 95% CI, 1.16-1.28) and having a hiatal hernia (OR, 2.70; 95% CI, 2.54-2.87).
Abnormal Secondary Peristalsis on FLIP Panometry In Patients With Normal Peristalsis on High-Resolution Manometry
ACG 2024 Plenary Session 3A, Abstract 38
To investigate the concordance of findings from functional luminal imaging probe (FLIP) panometry, which assesses secondary peristalsis, and high-resolution manometry (HRM), which assesses primary peristalsis, investigators, led by Rhea Fogla, MD, an internal medicine resident at NewYork-Presbyterian/Weill Cornell Medicine, in New York City, compared FLIP and HRM findings in patients who had both tests.
The retrospective cohort study included patients at a single center who had both 16-cm FLIP and HRM within a year of each other between June 2020 and June 2022. Patients also were required to have available data for maximum esophagogastric junction (EGJ) diameter and distensibility index at 60-mL fill. Panometry contraction findings were classified as normal, borderline, absent, spastic or impaired/disordered. HRM contraction findings were classified as normal, weak, premature, hypercontractile or failed.
Concordance was defined as normal findings for both, absent findings for both, borderline panometry and normal/weak HRM, or impaired/disordered/spastic panometry and premature/hypercontractile HRM.
In total, 121 patients were included in analyses. For the categories of panometry findings, the percentage of patients with concordant results based on HRM were:
- normal contractility on panometry (n=38): 82% had normal peristalsis based on HRM;
- absent contractility on panometry (n=38): 55% had some normal peristalsis—normal, weak or hypercontractile—on HRM;
- borderline contractility on panometry (n=32): 84% had normal or weak peristalsis on HRM; and
- spastic contractility on panometry (n=13): 61% had hypercontractile or premature peristalsis on HRM.
Discordance between panometry and HRM contraction patterns was seen in 26%. Those with discordance were more likely to have elevated integrated relaxation pressure on HRM (P<0.001). Discordance was not found to be associated with age, body mass index, or reduced or borderline EGJ opening on FLIP.
To Dr. Fogla and her co-investigators, “this study suggests that abnormal secondary peristalsis may be found in patients with normal primary peristalsis.” Thus, “an abnormal panometry should be followed by HRM, as both findings may be clinically relevant.”
High Economic Burden and Low Clinical Value of Sucralfate
ACG 2024 Plenary Session 3A, Abstract 40
Sucralfate is an FDA-approved short-term treatment for duodenal ulcers. Data are limited on its efficacy in this indication, as well as for off-label use for gastric ulcers, non-ulcer dyspepsia and reflux esophagitis. ACG guidelines recommend against its use for reflux disease, yet it is still a commonly prescribed medication.
{RELATED-VERTICAL}Researchers led by Neha Wadhavkar, MD, an internal medicine resident at Brown University, in Providence, R.I., used data in the ClinCalc database to evaluate cost and utilization trends of sucralfate and other medications used for upper GI conditions, including esomeprazole, famotidine, lansoprazole, omeprazole, pantoprazole and ranitidine, from 2013 to 2021. Data were available for all years for all drugs besides ranitidine, which was recalled in 2020.
In the most recent year of data, the price per prescription of these drugs ranged from $15.74 to $195, with sucralfate being the second most expensive ($119.40). Total annual cost of these drugs ranged from $51 million to $1 billion, with sucralfate costing $312 million.
Omeprazole was the most prescribed drug in each year of the study period, although it declined from a peak of 75 million annual prescriptions in 2015 to 54 million in 2021. Sucralfate prescribing increased from 1.8 million prescriptions per year in 2013 to 2.6 million in 2021.
Dr. Wadhavkar emphasized that these findings show that “sucralfate remains widely prescribed, despite unclear indications for its use in the 21st century,” and it has a “financial impact [that] is comparable to histamine-2 receptor antagonists and some proton pump inhibitors.”