Section Editor

Center for Advanced Therapeutic Endoscopy
Division of Gastroenterology and Hepatology
University of Rochester Medical Center
Rochester, New York
In recent years, outbreaks of infections with duodenoscope-associated multidrug-resistant organisms have increased awareness and concerns about the pitfalls in high-level disinfection protocols and traditional duodenoscope design. A call for innovative approaches to reduce the risk for transmission of multidrug-resistant organisms through duodenoscopes has led to the development of single-use duodenoscopes. As with any new technology, questions have been raised regarding the performance, safety, cost, feasibility of implementation, and environmental impact of these novel duodenoscopes.1 In this edition of Technology Spotlight, I had the opportunity to discuss this topic with Frank G. Gress, MD, MACG, FASGE, an expert endoscopist who shares his views with us in this review. We hope this information is useful to you in the care of your patients.

Icahn School of Medicine at Mount Sinai
New York, New York
Chief, Division of Gastroenterology and Hepatology
Mount Sinai South Nassau
Oceanside, New York
Dr. Kaul: Why has the single-use duodenoscope paradigm evolved?
Dr. Gress: The concept of single-use duodenoscopes (SUDs) evolved as a result of increasing concerns about infection control in the endoscopy unit and reprocessing of reusable duodenoscopes.2,3 In 2018, results from microbial culturing released by the FDA and CDC revealed contamination rates in reprocessed reusable duodenoscopes of up to 3.6% for low to moderate infectious organisms and up to 5.4% for highly infectious organisms.4-8
As a result of these data, the FDA advocated for the use of innovative duodenoscope designs to make reprocessing easier, more effective, or unnecessary altogether. This push for innovative duodenoscope design also led to new devices that were recently approved for use by the FDA.5
First, I think it is important to understand why the infection rate can be high in standard reusable duodenoscopes. The design of the duodenoscope is critical for visualization and maneuvering during the procedure and includes an important feature known as an “elevator” mechanism. This is critical for performing the endoscopic retrograde cholangiopancreatography (ERCP) procedure because it allows an endoscopist to accurately position and orient the catheter so they can perform the desired interventions at the papilla.
The elevator is in a recessed and relatively inaccessible space in the distal tip of the duodenoscope and contains a cable-actuated “lifting” mechanism that helps to guide and direct catheters and accessories (catheters, wires, balloons, etc) used during ERCP procedures through this channel into the pancreaticobiliary system. The elevator’s location makes manual cleaning and disinfecting of the duodenoscopes cumbersome.
Most ERCP-related infection transmission has been attributed to the complex elevator design and breaches related to cleaning, disinfecting, and/or drying protocols at that specific location.
Reusable duodenoscopes undergo standardized manual and automated reprocessing under strict FDA, CDC, Healthcare Infection Control Practices Advisory Committee, and manufacturer guidelines. In addition, repetitive reuse and reprocessing can lead to wear and tear, resulting in small scratches or cracks in critical components that can harbor bacteria, and over time, lead to biofilm formation.
As a result, duodenoscope-related infection rates with multidrug-resistant bacteria such as Pseudomonas aeruginosa, carbapenem-resistant Klebsiella pneumoniae, Enterobacter cloacae, and Escherichia coli have increased worldwide.4 Endoscopy-related infections can be life-threatening, with potential 1-month mortality rates of more than 20% in some cases.5
Dr. Kaul: Can you describe the basic concept and platforms for SUDs? What systems are currently available?
Dr. Gress: The basic concept and platform—like most medical devices in use today—is a single-use or disposable endoscopic instrument that can be used once and discarded. Of course, critical to this concept is that the performance of this device must be comparable to that of traditional reusable endoscopes, and it should be cost-effective in its design, manufacturing, and price point.
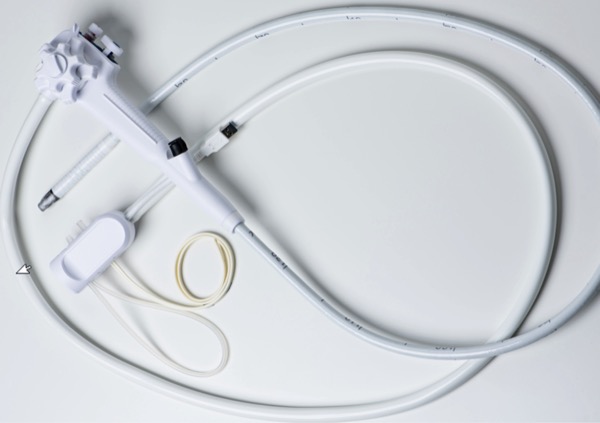
Two such systems are available and have been described and reported on.7-15 In 2019, the EXALT Model D (Boston Scientific) SUD, the first fully disposable duodenoscope, was approved by the FDA (Figures 1 and 2, page 40).7,8 The EXALT duodenoscope is specifically designed to mirror all features of established reusable duodenoscopes. The length of the duodenoscope is similar to that of reusable duodenoscopes (1,240 mm), it has a 4.2-mm diameter standard working channel and 4-way steering of the distal tip, and it has a locking mechanism to stabilize the distal tip for positioning and orientation. The image capture and air/water/suction buttons are in the same location and orientation as in reusable duodenoscopes. Furthermore, the elevator guidewire locking mechanism has a similar feel to that of reusable duodenoscopes.
In a randomized controlled study of 48 patients who underwent ERCP with EXALT, 46 successfully completed ERCPs (96%), with 2 patients (4.2%) requiring crossover to a reusable duodenoscope due to unsuccessful cannulation.8 Overall, there was no significant difference in the rate of adverse events between patients who underwent ERCP with the SUD versus a reusable duodenoscope.
The second available SUD is the aScope Duodeno (Ambu), which was FDA approved in 2020 (Figure 3, page 40).9,10 Ambu’s aScope is similar in concept to the EXALT, with a primary goal of decreasing and eliminating the chance of cross-contamination and infection.
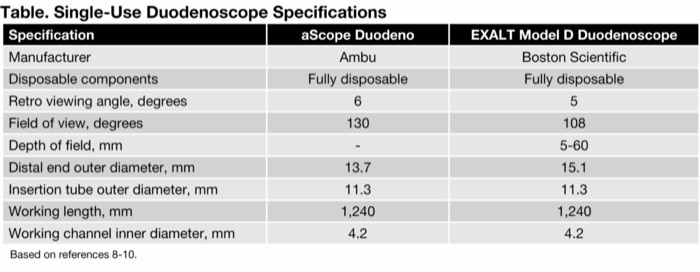
A recent study compared the safety and efficacy of both of these SUDs, reporting that both products were similar/comparable in their results.16 (See product specifications in the Table.)
| Table. Single-Use Duodenoscope Specifications | ||
| Specification | aScope Duodeno | EXALT Model D Duodenoscope |
|---|---|---|
| Manufacturer | Ambu | Boston Scientific |
| Disposable components | Fully disposable | Fully disposable |
| Retro viewing angle, degrees | 6 | 5 |
| Field of view, degrees | 130 | 108 |
| Depth of field, mm | - | 5-60 |
| Distal end outer diameter, mm | 13.7 | 15.1 |
| Insertion tube outer diameter, mm | 11.3 | 11.3 |
| Working length, mm | 1,240 | 1,240 |
| Working channel inner diameter, mm | 4.2 | 4.2 |
| Based on references 8-10. | ||
More recently, Ambu released a single-use upper endoscope and a single-use therapeutic upper endoscope. These instruments are designed to perform complete upper endoscopy exams. Currently, there are no available studies in the United States on these newer gastroscopes. However, potential applications for these instruments exist, with uses ranging from immunosuppressed or immunocompromised patients, critically ill or ICU patients, patients with GI bleeding, etc.
Dr. Kaul: What are the potential benefits as well as the potential disadvantages or limitations of SUDs?
Dr. Gress: The primary benefits of SUDs are reduced infection rates and decreased patient morbidity and mortality related to “superbug” infections.
The main disadvantage with the SUD paradigm is the added cost per procedure. Depending on an institution’s volume of procedures, the cost of switching over entirely to a SUD platform can be significant. There are also some concerns related to increasing the carbon footprint, but these must be weighed against the current waste generated by reprocessing, as well as the full-time equivalent employee work hours and infrastructure used in managing/maintaining reusable endoscopes.
In terms of limitations, the current literature has found that SUDs may require some design to be equal to current standard reusable instruments. Specifically, there have been some performance issues related to flexibility and maneuverability of the instruments as well as with the cannulation aspect, requiring crossover to a standard reusable instrument in a minority of cases. The optics and imaging sophistication also are being continually upgraded by the manufacturers. Although some of these issues may exist, there is obviously a huge price differential between the SUD and the traditional duodenoscope cost. Further enhancements may be limited by the need to adhere to a price point that is commercially viable.
Dr. Kaul: How does the performance of the two currently available SUDs compare?
Dr. Gress: There was a comparative study done between both available SUDs, with consecutive ERCP procedures performed by expert endoscopists from 9 academic centers.16 Performance ratings, procedure details, and information about adverse events were collected. The results were reported on 201 patients: 129 patients underwent ERCP with EXALT (mean age, 63 years; males, 51%) and 72 patients with the aScope Duodeno (mean age, 65 years; males, 42%). A majority of endoscopists in both groups had performed more than 2,000 ERCPs (71% EXALT, 93% aScope Duodeno). The procedural complexity for the ERCP cases performed were mostly grade 2 and 3 (grade 1, 35 cases [18%]; grade 2, 83 cases [41%]; grade 3, 65 cases [32%]; grade 4, 18 cases [9%]).
Technical success was 92% in both groups (EXALT, 119 vs aScope Duodeno, 66). Thirteen patients (10%) from the EXALT group and 16 (22%) from the aScope Duodeno group required conversion to a reusable duodenoscope.
On a scale of 1 to 5, the researchers rated the EXALT and aScope Duodeno scopes similarly in 4 areas: location and visualization quality, 2.31 versus 2.60; maneuverability based on papillary orientation, 1.38 versus 1.57; suction/air control, 1.48 versus 1.15; and elevator efficiency, 2.31 versus 2.34. No adverse events were related to the SUDs. The investigators concluded that both SUDs were comparable and recommended continued development and enhancements to these devices to improve maneuverability and clinical effectiveness.
Dr. Kaul: Are there any significant safety, functionality, or cost concerns with SUDs?
Dr. Gress: Overall, there are limited data in terms of safety and efficacy of SUDs. This is partly because SUDs are a relatively new technology and there are limited studies available evaluating the devices in clinical use. However, the current data evaluating outcomes and adverse events are promising.
Dr. Kaul: What patient populations are best suited for SUDs?
Dr. Gress: Ideally, these instruments are designed for patients who would be most affected by an infectious complication. These include immunosuppressed or immunocompromised patients, critically ill patients, and those in the ICU. Also, patients who need ERCP but are currently infected with multidrug-resistant bacteria are good candidates. Patient populations at low-volume or small endoscopy facilities that may not have the optimal staffing or equipment for endoscope reprocessing are also good candidates for the SUD platform.
Dr. Kaul: What is the environmental impact of SUDs?
Dr. Gress: This is a great question, but it is not entirely defined at this time. As the transition is made from reusable duodenoscopes to SUDs, it raises the questions of how this will impact the environment.
For standard reusable duodenoscopes, hazardous materials are used to clean the devices. Personnel are exposed to these hazardous chemicals and they must be stored. The appropriate disposal of hazardous materials can have serious implications if handling errors or prolonged exposure to chemicals occurs.
In one recent study, Namburar et al evaluated the amount of waste generated from endoscopy procedures from 2 US academic medical centers and determined that endoscopy generated approximately 2.1 kg of waste per procedure.17 Of this waste, 64% went to a landfill, 28% was biohazardous waste, and only 9% of this material was recycled. The total amount of waste from all endoscopic procedures in the United States annually is estimated to be about 18 million kg.
Separate from the physical waste produced, the energy consumption and greenhouse gas generated from the production of SUDs is an important factor, too. In a study by Le et al, these factors were considered by evaluating 3 different duodenoscopes: a reusable duodenoscope, duodenoscope with disposable endcaps, and SUD (EXALT Model).18 The study reported that performing ERCP with a SUD is associated with the release of 36.3 to 71.5 kg of carbon dioxide (CO2) equivalent, which is 24 to 47 times greater than that associated with use of a reusable duodenoscope (1.53 kg CO2) or a reusable duodenoscope with disposable endcaps (1.54 kg CO2). Most of the impact comes from its manufacturing, which accounts for 91% to 96% of its greenhouse gas emission.
In contrast, on its website, Ambu cites a study by Sørensen BL that concluded findings contrary to the above.19 The study found that if one set of personal protective equipment (PPE) is used in the cleaning process with reusable instruments, then reusable scopes had comparable or higher material and energy consumption and higher emission of CO2 equivalents.
Some potential limitations include the fact that this study was used to evaluate disposable bronchoscopes and not duodenoscopes. Also, the researchers concluded that as the assessed parameters are highly dependent on the cleaning protocols and use of PPE, they could not decisively conclude which bronchoscope would have the greater environmental impact.
As it pertains to SUDs, transparency in how materials are disposed of or recycled is important to determine if this transition is as safe or better than current practices. In a recent review, I found no journal articles, publications, or articles describing the appropriate disposal procedures.
Dr. Kaul: Are there competing technologies or devices in this space?
Dr. Gress: One alternative method to help reduce endoscope contamination and patient infection rates is a disposable elevator, inserted at the tip of the duodenoscope.20-22 The details of this modality are not within the scope of this discussion, but it is important to point out that this is a modification to the current reusable instruments, and essentially provides for a “disposable” elevator. There are no randomized controlled trials comparing SUDs to this technology, but if proven equivalent to SUDs relative to its infection prevention rate, this method may present a more cost-effective and economical alternative to the SUD platform.
The potential disadvantage to this product is that only the elevator is disposable, and the remainder of the duodenoscope is reusable after reprocessing. Therefore, theoretically, infection transmission is still possible from the reusable endoscope portion, and, in that regard, it may not be superior to a fully SUD endoscope.
Dr. Kaul: What are the capital and consumable costs, reimbursement rates, and insurance coverage?
Dr. Gress: Many factors affect annual costs to maintain performance operating standards with reusable duodenoscopes. Much of the revenue spent by hospitals is toward appropriate disinfection procedures, including cleaning equipment, chemicals, additional staff responsible for reprocessing of reusable duodenoscopes, surveillance testing performed on duodenoscopes to evaluate the effectiveness of the cleaning procedures, and service contracts with the duodenoscope manufacturers for repairs and maintenance.
The introduction of SUDs eliminates many of these traditional costs. However, the cost of purchasing a reusable versus SUD and the per-procedure cost are still a topic of debate. From available data, the cost of SUDs ranges between $797 and $4,400 (plus the processor), whereas the cost of a reusable duodenoscope is approximately $35,000, with an average life cycle of 3 to 7 years, depending on several factors (leasing vs purchasing, etc).
In 2019, Bang et al evaluated the cost per procedure by using an activity-based cost and financial model comparing low- and high-volume centers.23 Centers performing 50 or fewer ERCPs per year were designated as low volume, and centers with 150 ERCPs per year or greater were considered high volume. Factoring infection rates at an estimated 0.4% to 1%, the estimated per-procedure cost was lower for reusable scopes: reusable duodenoscopes, $612 to $1,362; SUDs in low-volume centers, $1,318 to $2,068; and SUDs in high-volume centers, $797 to $1,547.
However, the infection rate used in this study is a conservative measure compared with a recent meta-analysis published by Larsen et al, which indicated a rate of 1.21%.24
In 2021, Chahine et al published an abstract evaluating the cost of reusable duodenoscopes versus SUDs at a single high-volume center.25 The annual cost per procedure with the reusable duodenoscope was estimated to be $239.64 and their quoted price for the SUD was $1,950. Their cost evaluation did not factor in duodenoscope-related infections, which can increase the cost per procedure. Moreover, this was a quoted price that may or may not be lowered if the SUDs were purchased in bulk or through a contractual arrangement.
Travis et al conducted a multicenter study in 2020 analyzing the totality of investment costs required per ERCP for reusable duodenoscopes.26 They determined that for low-volume centers (<350 ERCPs/year) and high-volume centers (>350 ERCPs/year), the overall costs when considering all factors were $1,220.58 to $2,685.76 and $1,110.29 to $1,338.78, respectively. They also calculated the weighted average per procedure cost, which ranged between $1,283.93 and $1,378.29 when applying an infection rate of 1% and 1.2%, respectively.
These values are significantly higher than those in some of the previously mentioned studies, but the post-endoscopy infection rate, which significantly affects cost per ERCP, was more in line with the meta-analysis published by Larsen et al in 2020.24
As reimbursement patterns evolve, the overall cost per procedure for SUDs is estimated to rival or be superior to those with reusable devices. However, this can take some time since further studies will be needed to support cost-effectiveness of SUDs.
In addition, the manufacturers of SUDs are unlikely to be able to meet the demand for complete replacement of reusable duodenoscopes throughout the United States since the manufacturing capability cannot simply keep up with the total number of ERCP procedures being performed annually.
For now, it is critical to identify the selected patient groups or ideal patient populations that would benefit the most from SUDs.
Drs. Gress and Kaul are members of the Gastroenterology & Endoscopy News editorial board. They reported no relevant financial disclosures.
References
- Lee T, et al. Gastroenterol Hepatol. 2022;18(5):248-291.
- Forbes N, et al. Clin Transl Gastroenterol. 2020;11(8):e00214.
- Spach DH, et al. Ann Intern Med. 1993; 118(2):117-128.
- Trindade AJ, et al. Gastrointest Endosc. 2021;93(5):997-1005.
- Ross AS, B et al. Gastrointest Endosc. 2020;91(2):396-403.
- FDA. Use Duodenoscopes with Innovative Designs to Enhance Safety: FDA Safety Communication. Published June 30, 2022. Accessed September 5, 2024. fda.gov/medical-devices/safety-communications/use-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication
- Ehrlich D, et al. Expert Rev Med Devices. 2021;18(5):421-427.
- Boston Scientific. EXALT? Model D Single-use Duodenoscope. Accessed September 5, 2024. bostonscientific.com/products/DE/en/all-products/gastroenterology-products/single-use-imaging/exalt-model-d/p/P0051#Product-0
- Reddot. Single-Use Duodenoscope Ambu® aScope? Duodeno. Accessed September 5, 2024. red-dot.org/ko/project/ambur-ascopetm-duodeno-49936
- Ambu. Ambu® aBox? Duodeno. Accessed September 5, 2024. ambuusa.com/endoscopy/gastroenterology/duodenoscopes/product/abox-duodeno
- Bang JY, et al. Gut. 2021;70(5):838-844.
- Slivka A, et al. Gastrointest Endosc. 2021;94(6):1046-1055.
- Christensen M, et al. Gastrointest Endosc. 2004;60(5):721-731.
- Muthusamy VR, et al. Clin Gastroenterol Hepatol. 2020;18(9):2108-2117.e3.
- Holzwanger EA, et al. VideoGIE. 2020;5(12):628-629.
- Shahid HM, et al. J Clin Gastroenterol. 2023;57(8):798-803.
- Namburar S, et al. Gut. 2022;71(7):1326-1331.
- Le NNT, et al. Gastrointest Endosc. 2022;96(6):1002-1008.
- Sørensen BL, et al. Am J Environ Prot. 2018;7(4):55-62.
- Forbes N, et al. BMC Gastroenterol. 2020;20(1):64.
- Pasricha PJ, et al. Gastrointest Endosc. 2020;92(1):199-208.
- Jin P, et al. Gastrointest Endosc. 2019;89(6):AB237-AB238.
- Bang JY, et al. Gut. 2019;68(11):1915-1917.
- Larsen S, et al. EClinicalMedicine. 2020;25:100451.
- Chahine A, et al. Gastrointest Endosc. 2021;93(6):AB43-AB44.
- Travis HS, et al. Pharmacoeconomics: Open Access. 2020;5(1). doi:10.37421/pe.2020.5.125