Colonoscopy for colorectal cancer screening may not be a thing of the past yet, but two noninvasive screening tests are touting new data showing rates of sensitivity and specificity that are gaining on the gold-standard screening modality. New data on the cell-free DNA blood-based screening test Shield (Guardant Health) put it in the level of other noninvasive screening tests, researchers said, and improved metrics—including fewer false positive scores—of the next-generation of the multitarget stool DNA test Cologuard (Exact Sciences) show the test’s benefit for screening average-risk adults.
Cell-Free DNA Assay: 83% Sensitivity for CRC
The cfDNA test Shield demonstrated an 83% sensitivity and 90% specificity for clinically relevant CRC in a large population of individuals at average CRC risk, according to results from the ECLIPSE clinical validation trial.
“This is the first blood-based test with performance comparable to current guideline-recommended noninvasive CRC screening options,” said investigator Daniel Chung, MD, the director of the Hi-Risk Gastrointestinal Cancer Clinic at Massachusetts General Hospital, in Boston.
The assay under investigation is based on analysis of DNA shed from tumor cells and circulating freely throughout the blood plasma compartment. The analysis comprises genotyping of mutations associated with CRC, tumor-specific DNA methylation patterns and DNA fragmentation patterns. “These signals are integrated to produce a final actionable binary result that is read out as either normal or abnormal,” Dr. Chung explained.
The ECLIPSE study evaluated the sensitivity and specificity of the test in detecting CRC and precursor lesions in 7,861 average-risk individuals at least 45 years of age (mean age, 60 years). The study group was reported as slightly more women (54%) than men, and race was cited as predominantly white (79%), with smaller percentages of Black/African American (12%), Asian (7%) or other (2%) individuals; 12% of the study group identified as Hispanic/Latino. Enrollees presented for screening colonoscopy at 265 clinical sites across the United States between 2019 and 2022 and provided whole blood samples that were analyzed for cfDNA by technicians who were blinded to colonoscopy outcome.
Colonoscopy outcomes were classified based on the most advanced lesion: CRC; advanced precancerous lesion (APL), which included sessile serrated lesions larger than 10 mm and conventional advanced adenomas; non-APL; no precancerous lesion; or incomplete procedure. The overall objective was to test the performance of this cfDNA assay compared with a reference colonoscopy with its associated histopathology.
Results In Line With Other Noninvasive Modalities
Colonoscopies revealed 65 CRCs; 1,116 APLs; and 2,166 non-advanced precancerous lesions. Neoplasia was not detected in 5,514 patients.
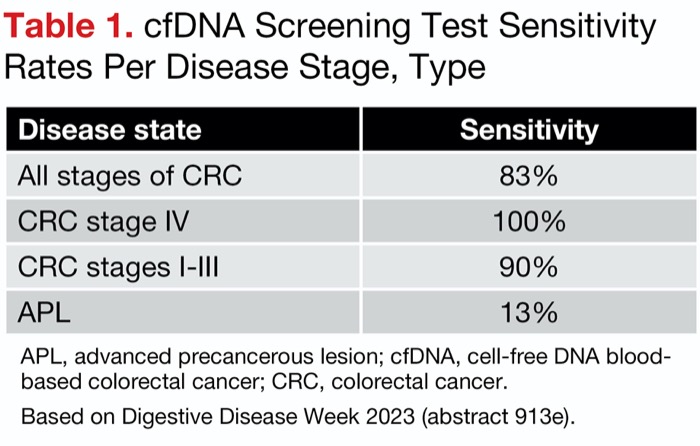
Among the 65 CRCs, 54 tested positive with the cfDNA assay, yielding an overall sensitivity of 83% (95% CI, 72%-90%) Sensitivity results per cancer stage are shown in Table 1. The test also showed similar specificities for both the absence of any cancer or APL as well as negative test results (absence, 90%; 95% CI, 89%-90%; negative test, 90%; 95% CI, 89%-91%).
| Table 1. cfDNA Screening Test Sensitivity Rates Per Disease Stage, Type | |
| Disease state | Sensitivity |
|---|---|
| All stages of CRC | 83% |
| CRC stage IV | 100% |
| CRC stages I-III | 90% |
| CRC stage III | 100% |
| CRC stage II | 100% |
| CRC stage I | 55% |
| APL | 13% |
| APL, advanced precancerous lesion; cfDNA, cell-free DNA blood-based colorectal cancer; CRC, colorectal cancer. Based on Digestive Disease Week 2023 (abstract 913e). | |
According to Dr. Chung, who presented the late-breaking abstract at Digestive Disease Week 2023 (abstract 913e), these results align with other guideline-recommended noninvasive screening modalities, with overall sensitivities ranging from 74% with fecal immunochemical testing to 92% with the multitarget stool DNA test (N Engl J Med 2014;370[14]:1287-1297).
During the discussion, Dr. Chung noted that this cfDNA assay focuses on markers that are highly specific for CRC. “Markers that we need to detect polyps and adenomas are probably going to be different,” he said.
However, with the data they have right now, Dr. Chung said he believes cfDNA assays could help increase CRC screening adherence. “Ultimately, the success of a screening test will depend upon the rate of uptake and adherence,” he said. “We have presented results under real-world clinical conditions, using this specific cfDNA assay over the past year, and the adherence rates (85%-96%) are consistently higher” than have been reported for other noninvasive modalities (43%-71%) (JAMA 2021;325[19]:1978-1998; Ann Intern Med 2016;164[7]:456-463; Endoscopy 2011;43[12]:1059-1086).
“If we consider this high adherence rate in conjunction with high sensitivity, this blood-based cfDNA approach has the potential to be a highly effective option for screening in the population,” he predicted.
Better Identification of High-Risk Adenomas Needed
Two CRC screening experts interviewed by Gastroenterology & Endoscopy News said the cfDNA test evaluated in this study still falls a bit short as a future screening modality.
According to David Lieberman, MD, a professor of medicine and the head of the Division of Gastroenterology and Hepatology at Oregon Health & Science University, in Portland, the study demonstrated “good proof of principle for very effectively” identifying patients with stage II, III and IV CRC, “for which there will be some benefit.” But its sensitivity for detecting stage I cancers was just 55% and for advanced adenomas 13%, he pointed out, noting that a modality that prevents cancers must better target its precursors.
Thomas Imperiale, MD, the Lawrence Lumeng Professor of Gastroenterology and Hepatology at Indiana University School of Medicine, in Indianapolis, was interested in knowing the lower 95% confidence limit for the sensitivity point estimate of 72% found for curable-stage (I and II) cancers. Further, he expressed concern that the 13% sensitivity for advanced adenomas may not reflect the test’s accuracy in identifying high-risk lesions. This point estimate “may have been due mostly or nearly completely to serendipity, which would occur if a false-positive result for which colonoscopy was performed ‘found’ an advanced adenoma by chance,” he said.
Altogether, the test will not soon replace other noninvasive testing, Dr. Lieberman concluded. While he considers the current stool-based tests to also be “a little disappointing” in their ability to detect advanced adenomas, at this point they are still “superior,” he said.
Shield is available for eligible individuals by prescription only through healthcare professionals.
Next-Gen Multitarget Stool DNA Test Has Improved Specificity, Sensitivity
The sensitivity and specificity results for the next-generation mt-sDNA test Cologuard show that a new version of the test has improved efficacy for detecting colorectal cancer and its precursors.
“These new results suggest an improved performance of the mt-sDNA test, which strengthens the case for molecular stool-based testing in preventing colorectal cancer,” said principal investigator Dr. Imperiale.
The BLUE-C trial is a multicenter, prospective study of more than 20,000 adults 40 years of age and older, designed to evaluate the performance of the next-generation mt-sDNA test. Using colonoscopy as the reference method, the study directly compared the test with commercial fecal immunochemical testing (FIT).
“Exact Sciences saw the need to try to improve upon how the test performed, especially its specificity,” Dr. Imperiale told Gastroenterology & Endoscopy News. “The false-positive rate is considered high for a screening test, and [also] in comparison to FIT [rates]. Exact also wanted to improve upon some of the test’s processing measures. They went back to the drawing board and were very methodical about finding markers that could improve the test performance—increasing specificity but also maintaining or even improving sensitivity.”
The resulting BLUE-C study is one of the largest colorectal cancer screening trials ever conducted, according to the company. The study population reflects the racial and ethnic composition of the United States as determined by the 2020 census. The topline results for the next-generation test were provided in a press release and in interviews with Gastroenterology & Endoscopy News. No date has been set for peer-reviewed publication of the findings.
Improving Sensitivity, Reducing False-Positive Rates
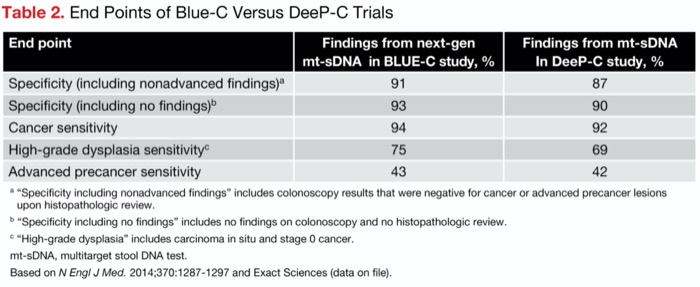
The modification of the test—specifically, the application of three new DNA methylation markers—resulted in an improvement in all the study’s metrics, compared with the results of the original mt-sDNA test in the registrational DeeP-C study (N Engl J Med 2014;370:1287-1297) (Table 2).
| Table 2. End Points of Blue-C Versus DeeP-C Trials | ||
| End point | Findings from next-gen mt-sDNA in BLUE-C study, % | Findings from mt-sDNA In DeeP-C study, % |
|---|---|---|
| Specificity (including nonadvanced findings)a | 91 | 87 |
| Specificity (including no findings)b | 93 | 90 |
| Cancer sensitivity | 94 | 92 |
| High-grade dysplasia sensitivityc | 75 | 69 |
| Advanced precancer sensitivity | 43 | 42 |
a “Specificity including nonadvanced findings” includes colonoscopy results that were negative for cancer or advanced precancer lesions upon histopathologic review. b “Specificity including no findings” includes no findings on colonoscopy and no histopathologic review. c “High-grade dysplasia” includes carcinoma in situ and stage 0 cancer. mt-sDNA, multitarget stool DNA test. Based on N Engl J Med. 2014;370:1287-1297 and Exact Sciences (data on file). | ||
“The result was a mt-sDNA test that was a lot better—with a 4% absolute increase in specificity, which represents a 30% reduction in false-positive tests,” Dr. Imperiale noted. “Given its sensitivity, it bests single-application FIT for detection of advanced adenomas and cancers. Average-risk adults with no strong family history of CRC, which is approximately 85% of the population, and no previous colorectal neoplasia qualifies for this improved test.”
Jeffrey Meyerhardt, MD, the co-director of the Colon and Rectal Cancer Center at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, in Boston, said he found the results cautiously optimistic. “It is encouraging to see improvements in sensitivity and specificity for the next generation of mt-sDNA testing, improving detection rates for those who have colorectal cancer and reducing false-positive rates. However, the sensitivity for precancer polyps still remains modest, and thus use of mt-sDNA solely limits detection of lesions before they become cancerous, which is one way to prevent colorectal cancer.”
—Caroline Helwick
Dr. Chung reported a financial relationship with Guardant. Dr. Imperiale reported that his institution receives grant support from Exact Sciences. Dr. Meyerhardt reported a financial relationship with Merck.
{RELATED-HORIZONTAL}