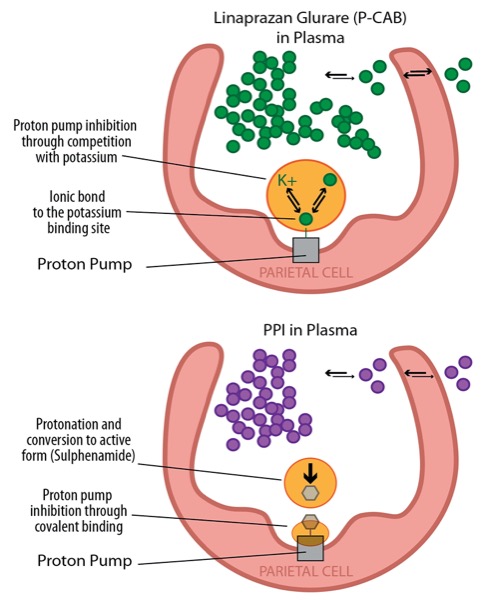
CHICAGO—Two potassium-competitive acid blockers were associated with a potential healing advantage for erosive esophagitis over a standard dose of a proton pump inhibitor in separate randomized, double-blind trials.
In both studies, the rate of EE healing was higher with the P-CABs than a 30-mg once-daily dosage of the PPI lansoprazole, and in one study, the healing was faster. But the trials tested noninferiority, and experts say larger trials are needed to confirm the role of these agents.
In one study, a double-blind, multicenter phase 4 trial, 50 mg of tegoprazan was associated with numerically higher healing rates than 30 mg of lansoprazole, but the trial was not powered to show superiority. Thus, principal investigator Cheol Min Shin, MD, from the Department of Gastroenterology at Asan Medical Center, in Seoul, South Korea, characterized 50 mg of tegoprazan as “possibly more effective” than 30 mg of lansoprazole.
Similarly, the P-CAB linaprazan glurate was associated with numerically higher healing than 30 mg of lansoprazole across multiple end points in the second study, a phase 2 trial, but senior author David Armstrong, MD, a professor of gastroenterology at McMaster University, in Hamilton, Ontario, said it was not a comparative trial. Rather, the PPI served as a benchmark for evaluating EE healing.
No P-CAB is licensed in the United States for EE. The only P-CAB with any U.S. indication is vonoprazan (Voquezna, Phathom), but its approval is only for use within a combination product for the eradication of Helicobacter pylori. Several P-CABs are approved outside of the United States and Europe. Vonoprazan has been approved in Japan for acid-related gastrointestinal diseases since 2015. In South Korea, where three P-CABs are approved for clinical use, new guidelines have characterized them as being equivalent to PPIs, according to Dr. Shin.
Tegoprazan Noninferior to PPI for EE
At Digestive Disease Week 2023, Dr. Shin presented the results of the phase 4 trial, which was designed to test noninferiority of tegoprazan to a PPI in 213 patients with EE (abstract 941). Healing at two and four weeks was evaluated as a secondary end point to compare speed of healing.
At four weeks, the healing rates were 94.2% and 86.2% for patients randomized to 50 mg of tegoprazan and 30 mg of lansoprazole, respectively. Owing to a higher rate of healing for the P-CAB, noninferiority of tegoprazan to lansoprazole was met at a level of high significance (P<0.00001).
The rates of every outcome measured favored the P-CAB, including endoscopically confirmed healing in the most severe grades of esophagitis (Los Angeles grades C and D) and relief of heartburn in a patient-reported outcome tool.
Consistent with prior studies, EE healing appeared to be faster when assessed at two weeks, even though the advantage over lansoprazole (87.5% vs. 82.6%) did not reach statistical significance, according to Dr. Shin, who said he believes larger trials with greater power potentially will confirm differences.
Healing Better With Linaprazan In Dose-Finding Study
In the phase 2, dose-ranging study of the P-CAB linaprazan glurate, patients were randomized to one of four P-CAB doses (25, 50, 75 and 100 mg) twice daily or 30 mg of once-daily lansoprazole (a dummy pill in the lansoprazole group maintained the blind).
For Los Angeles grade A and B EE, 100% were healed on any dose of linaprazan glurate at four weeks versus 74% of those randomized to lansoprazole, according to Dr. Armstrong, who presented the data at DDW 2023 (abstract 940). For grades C and D, healing on the most effective dose of linaprazan glurate was 74%, versus 33.3% for lansoprazole.
During the discussion, some attendees criticized the study design for comparing a once-daily PPI with twice-daily P-CABs, suggesting that a twice-daily dosage or a 40-mg dose of esomeprazole would have been a more appropriate comparison. However, Dr. Armstrong reemphasized that this was not a comparative study. Instead, the goal was to consider appropriate doses for healing of EE for a planned phase 3 linaprazan glurate EE trial.
Rapid Onset, Lack of Food Effects Are Potential Benefits
Others who have been involved in the study of P-CABs also see a potential role for these agents as an alternative to PPIs, particularly if large phase 3, multicenter trials that include centers in the United States provide a more rigorous demonstration of their efficacy and safety.
“P-CABs appear to offer some advantages over PPIs in the management of [gastroesophageal reflux disease],” reported Colin Howden, MD, the chief of the Division of Gastroenterology at University of Tennessee Health Science Center, in Memphis. “Among these are the more rapid healing of EE, especially severe grades, and superior maintenance of EE healing.”
Dr. Howden, who has been a lead author or co-author of several studies and review articles addressing the potential use of P-CABS, agreed that the data support a more rapid onset of action for drugs in this class than for PPIs. He also noted that P-CABs, unlike PPIs, do not need to be timed with meals to maximize their effect. “For GERD patients who do not have EE, [the rapid onset and lack of food effect] may enable the future use of P-CABs on an ‘as-needed’ basis,” he said, although he agreed that large registration trials are needed to move these drugs forward.
—Ted Bosworth
Dr. Armstrong reported financial relationships with A.I. VALI, Cinclus, NestlÉ, Phathom and Takeda. Dr. Howden reported financial relationships with Phathom and Takeda. Dr. Shin reported no relevant financial disclosures.
{RELATED-HORIZONTAL}