CHICAGO—Modulation of the gut microbiome using a novel probiotic–prebiotic combination formula helped relieve symptoms related to post-acute COVID-19 syndrome in a randomized, blinded, placebo-controlled trial presented as a late-breaking abstract at Digestive Disease Week 2023.
“This is the first randomized controlled trial showing that modulation of the gut microbiome with a novel microbiome formula alleviates gastrointestinal and neuropsychiatric symptoms of post-acute COVID-19 syndrome,” said lead researcher Raphaela Iris Lau, MSc.
A Synbiotic Approach
The study, conducted at the Chinese University of Hong Kong, included 463 patients with symptoms of long COVID who received six months of treatment with an active microbiome product that was a combination of probiotic and prebiotic material, called a synbiotic (SIM01) (abstract 913a).
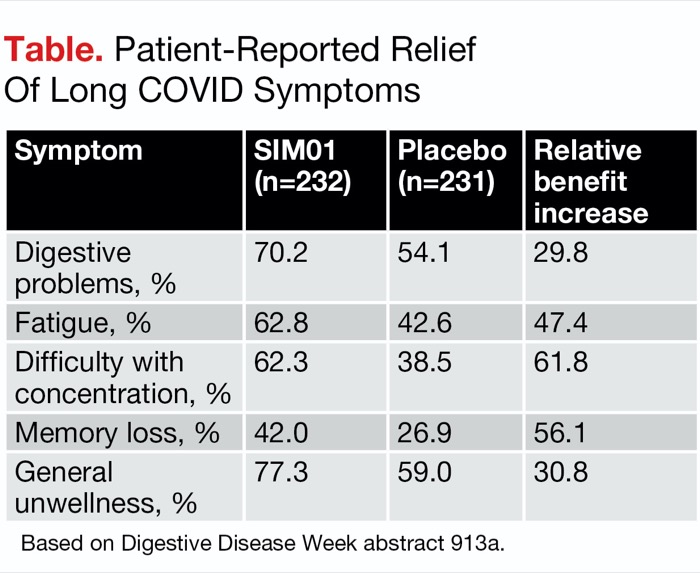
Compared with blinded controls, the actively treated patients were likely to report improvement in various symptoms, including digestive problems, fatigue, difficulty with concentration, memory impairment and “general unwellness,” reported Ms. Lau, a PhD fellow at the university. For example, improvements in digestive complaints were observed in 70.2% of SIM01 recipients compared with 54.1% of controls, who received vitamin C, Ms. Lau reported.
Amir Zarrinpar, MD, PhD, an assistant professor in the Division of Gastroenterology at the University of California, San Diego, who has conducted microbiome research, told Gastroenterology & Endoscopy News that he was encouraged by the findings. “This study presents exciting preliminary evidence that the gut microbiome may play a role in managing long COVID symptoms. The results are indeed provocative, showing significant reduction in several major symptoms with the use of the microbiome product,” he continued. “This study provides a promising starting point, and I am excited to see where subsequent research in this area will lead us.”
Developed by Ms. Lau’s co-investigators, SIM01 is an oral, microencapsulated formulation of three lyophilized Bifidobacteria and three prebiotics shown to be beneficial for the growth of these bacteria, offering a total of 20 billion colony-forming units per daily dose. In a previous randomized, double-blind, placebo-controlled trial, SIM01 was shown to significantly reduce adverse health outcomes of COVID-19 in elderly patients and patients with type 2 diabetes (Nutrients 2023;15[8]:1982). In a proof-of-concept study, SIM01 also hastened antibody formation against SARS-CoV-2, reduced nasopharyngeal viral load and pro-inflammatory immune markers, and restored gut dysbiosis in patients hospitalized with COVID-19 (J Gastroenterol Hepatol 2022;37[5]:823-831).
In the current study, the investigators selected adults diagnosed with COVID-19 presenting with at least one symptom that had persisted for at least four weeks after their original diagnosis. The mean age was 49 years, and median time since diagnosis was four months.
Of note, only 12% of the placebo arm and 16% of the active treatment arm reported a moderate, severe or critical COVID-19 infection. More than two-thirds of patients were infected with the omicron variant, while less than one-third contracted an earlier variant. “We found the prevalence of long COVID to be quite similar [for the variants],” Ms. Lau said.
In the triple-blinded trial, patients were randomly assigned to receive SIM01 or vitamin C daily for six months. The primary outcome was alleviation of long COVID symptoms by six months compared with baseline, as assessed by a structured, interviewer-administered, 14-item symptom questionnaire. Approximately 83% of each group reported at least 90% compliance with therapy.
“We assumed the rate of developing the primary outcome—alleviation of long COVID symptoms by month 6—would be 70% on active intervention and 50% on placebo,” Ms. Lau said.
Indeed, the findings matched the researchers’ expectations. “For all symptoms assessed, the SIM01 group had higher proportions of subjects with alleviation,” Ms. Lau said. After Bonferroni correction, the differences between the groups were significant for five symptoms: digestive problems, fatigue, difficulty with concentration, memory loss and general unwellness (P<0.0036) (Table).
| Table. Patient-Reported Relief Of Long COVID Symptoms | |||
| Symptom | SIM01 (n=232) | Placebo (n=231) | Relative benefit increase |
|---|---|---|---|
| Digestive problems, % | 70.2 | 54.1 | 29.8 |
| Fatigue, % | 62.8 | 42.6 | 47.4 |
| Difficulty with concentration, % | 62.3 | 38.5 | 61.8 |
| Memory loss, % | 42.0 | 26.9 | 56.1 |
| General unwellness, % | 77.3 | 59.0 | 30.8 |
| Based on Digestive Disease Week abstract 913a. | |||
The relief of digestive complaints was broad. Significant reductions in severity scores were observed for diarrhea, constipation, abdominal pain, epigastric pain, bloating, vomiting and acid reflux, all as self-reported on an eight-item gastrointestinal symptom severity scale, she added. Adverse events were reported by about one-fourth of each arm and were unlikely to be related to the study product, she said.
Clarification Needed
Although the study is encouraging, the findings need more clarification, Dr. Zarrinpar commented. “The precise content and mechanism of action of SIM01 remain unclear” and the study “was not registered in ClinicalTrials.gov, which is the biggest red flag for me” because it “makes it challenging to verify its objectives and power analysis,” he noted.
Clinicians should “balance optimism with a healthy skepticism when communicating with their patients,” about the findings, Dr. Zarrinpar said. “It’s crucial to underscore that while this study does provide some evidence of potential benefit, we still need more comprehensive and rigorous scientific research to definitively confirm the effectiveness of SIM01 in treating long COVID.”
Dr. Zarrinpar told Gastroenterology & Endoscopy News that “given these points, [clinicians] might tell their patients that while the results of this study are intriguing, it would be premature to make any treatment decisions based on it alone.”
—Caroline Helwick
Ms. Lau reported no relevant financial disclosures. Dr. Zarrinpar reported that he is the co-founder and acting chief medical officer of Endure Biotherapeutics.
{RELATED-HORIZONTAL}