WASHINGON—The investigational agent bepirovirsen appears most effective in reducing hepatitis B viral levels for patients with chronic infection when given at a dose of 300 mg for 24 weeks, according to results of a phase 2b trial.
At that dosage, 9% to 10% of patients treated with the antisense oligonucleotide bepirovirsen (GSK), with or without stable nucleoside or nucleotide analog (NA) therapy, achieved the primary study outcomes of hepatitis B surface antigen (HBsAg) and HBV DNA loss for 24 weeks after the end of treatment.
The trial’s investigators also found that a second dosage plan of 200 mg for 12 weeks followed by 150 mg for 12 weeks was efficacious, with 9% of the patients in that study arm who received NA therapy reaching the primary end point. The effect was reduced when patients at this dosage did not receive NA therapy.
Bepirovirsen is designed to target the pre-genomic HBV RNA and all HBV mRNAs, resulting in a decrease in viral proteins, said lead study investigator Man-Fung Yuen, MD, PhD, DSc, the chair and chief of the Division of Gastroenterology and Hepatology at the University of Hong Kong.
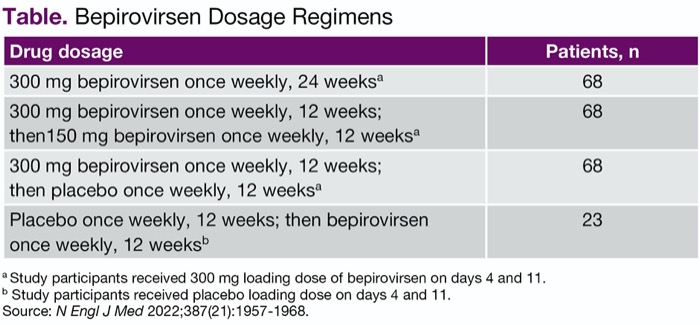
In the multicenter, randomized, investigator-unblinded B-Clear study, patients were randomized 3:3:3:1 to one of four treatment arms to receive up to 300 mg of bepirovirsen, administered as two subcutaneous injections weekly for 12 or 24 weeks (Table). The study was conducted at 123 sites in 22 countries.
| Table. Bepirovirsen Dosage Regimens | |
| Drug dosage | Patients, n |
|---|---|
| 300 mg bepirovirsen once weekly, 24 weeksa | 68 |
| 300 mg bepirovirsen once weekly, 12 weeks; then150 mg bepirovirsen once weekly, 12 weeksa | 68 |
| 300 mg bepirovirsen once weekly, 12 weeks; then placebo once weekly, 12 weeksa | 68 |
| Placebo once weekly, 12 weeks; then bepirovirsen once weekly, 12 weeksb | 23 |
a Study participants received 300 mg loading dose of bepirovirsen on days 4 and 11. b Study participants received placebo loading dose on days 4 and 11. Source: N Engl J Med 2022;387(21):1957-1968. | |
All participants had long-term HBV infection and were on chronic NA therapy for at least six months, with alanine aminotransferase (ALT) levels at most two times the upper limit of normal, HBV DNA less than 90 IU/mL and HBsAg greater than 100 IU/mL. Some 73% of participants were male and 52% were Asian. The majority of the patients (69%) were hepatitis B e antigen (HBeAg)-negative and had an HBsAg level greater than 3 log10 IU/mL (72%). Participants were followed for up to 55 weeks, with a 24-week treatment period and 24-week follow-up period.
Higher Dose More Effective
The team presented results on 227 patients with chronic HBV infection and on stable NA therapy (abstract 5015) as well as results on those not taking NA therapy (abstract 5022) at The Liver Meeting 2022. Full results of the trial in 457 participants were published in The New England Journal of Medicine (2022;387[21]:1957-1968).
More patients achieved the primary end point in the higher dosage arms than in the lower dosage arms, and no patients in the placebo arm reached the end points. A greater proportion of patients with low versus high baseline HBsAg levels achieved the primary outcome (a difference of 16% vs. 6% in arm 1). The primary outcome was achieved in a similar proportion of HBeAg-negative and -positive patients (10% vs. 6% in arm 1).
Treatment was discontinued in 13 patients (6%), and eight patients (4%) had an adverse effect (AE) that led to discontinuation. The most common AEs were injection site reactions (64%), pyrexia (14%) and increase in ALT (11%).
Larger trials are needed to assess the drug’s efficacy and safety, the investigators noted.
Clearing Virus Without Relapse
The moderator of the conference session, Jay Hoofnagle, MD, agreed, saying “these results are very promising, but require long-term assessment of whether they are lasting and result in changes in long-term outcome of this disease.”
New approaches to therapy for HBV are needed because of “the unique complexity of HBV replication and its ability to persist despite profound viral suppression,” Dr. Hoofnagle, the director of the Liver Disease Research Branch of the Division of Digestive Diseases and Nutrition at the National Institute of Diabetes and Digestive and Kidney Diseases, noted in an accompanying editorial in The New England Journal of Medicine (2022;387[21]:1996-1998).
Most patients who receive NA therapy remain positive for HBsAg and are at risk for relapse, which can be severe and, although rare, even fatal if therapy is stopped, he noted.
“What is needed is a regimen that would lead to clearance of both HBV DNA and HBsAg and allow for withdrawal of all therapy without a risk of relapse,” he added.
Further pursuit of bepirovirsen is warranted, Dr. Hoofnagle told Gastroenterology & Endoscopy News, but critical questions remain as to whether HBsAg negativity will persist beyond 24 weeks, when NA therapy could safely be stopped, what other factors predict response, and whether long-term complications of chronic HBV will be prevented. “Thus, these changes in virologic markers are ‘surrogate’ markers for the avoidance of the long-term clinical outcomes—liver cancer and end-stage liver disease.”
—Karen Blum
Dr. Hoofnagle reported no relevant financial disclosures. Dr. Yuen reported financial relationships with numerous companies, including GSK, the manufacturer of bepirovirsen.
{RELATED-HORIZONTAL}