Boston, Mass.
The American College of Gastroenterology recently released a new clinical guideline on the diagnosis and management of subepithelial lesions of the gastrointestinal tract (Am J Gastroenterol 2023;118[1]:46-58). GEN’s Sarah Tilyou spoke with lead author Brian C. Jacobson, MD, MPH, FACG, AGAF, FASGE, a gastroenterologist at Massachusetts General Hospital and an associate professor of medicine at Harvard Medical School, in Boston, about the impetus for the guideline and what it means to GI practice.
GEN: What prompted the guideline?
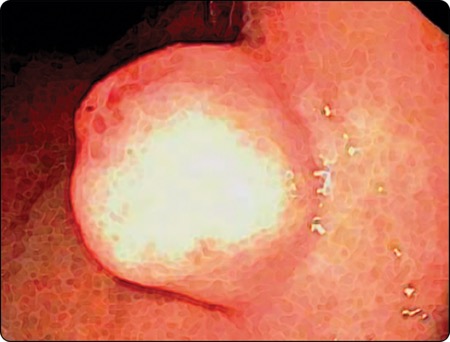
Dr. Jacobson: This was a guideline about a topic that had been addressed by other organizations, such as the American Society for Gastrointestinal Endoscopy (Gastrointest Endosc 2017;85[6]:1117-1132) and the European Society of Gastrointestinal Endoscopy (Endoscopy 2022;54[4]:412-429), but had not been addressed before by the ACG. The ACG thought this was important because endoscopists fairly frequently find incidental small bumps in the wall of the stomach, esophagus or rectum and have questions about how to manage them.
Another rationale for developing new guidelines is that there are now more therapeutic options available for advanced endoscopists to use to resect some of these lesions in the wall of the GI tract. So, it was a good idea to review the data and see whether any particular therapies that have emerged are superior. Following the GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology, we found that there were not enough robust, well-done studies to have strong definitive statements. So, the guideline includes a fair number of conditional statements with recommendations or suggestions rather than hard-and-fast rules. So, in a sense, it’s also a call for more rigorous studies in this field to help us learn whether there are truly superior ways of evaluating and managing these lesions.
GEN: What’s new in the guideline that clinicians need to know?
Dr. Jacobson: A few new things stood out.
With respect to diagnosis, we found that endoscopic ultrasound really was a superior way to yield a diagnosis compared with standard endoscopy or cross-sectional imaging, such as CT scans. In addition, getting a sample of tissue in some way was very important for yielding a definitive answer. We explored various biopsy options used with endoscopic ultrasound, and we found that as long as you have a pathologist to help evaluate your sample in real time during the procedure, it doesn’t matter if you are doing fine needle aspiration or fine needle biopsy. But if you do not have someone to help assess how good your specimens are when you’re doing the biopsy, you really want to try to do fine needle biopsy to make sure you had a good core of tissue.
In addition, we really tried to stress that sometimes you don’t need an endoscopic ultrasound or further evaluation. For example, if the patient is presenting with obvious blood loss from a lesion, and you’ve seen it with your standard endoscope, you can simply tattoo the site and send the patient to a surgeon to remove the lesion, because they’re symptomatic. There’s no need to extend testing or prolong treatment. That was important for a GI society to come out and state.
Another thing that we touched on that may be a little controversial is bite-on-bite, or tunnel biopsy, where an endoscopist will see a lesion that’s clearly in the wall and not on the surface. In this case, they’ll take a biopsy, then biopsy the biopsy site, and perhaps biopsy the site again—really digging in, hoping to reach the layer under the surface where this lesion is located. The literature did not support this approach as being reliable. This is partly because if the lesion is in the muscularis propria, you won’t get it via tunnel biopsy anyway. The other reason is that some of these lesions may appear to be solid but actually are blood vessels. So, we felt that because the data were lacking, endoscopists would be better off doing endoscopic ultrasound and getting tissue through that method, so they can assess whether the lesion is a blood vessel or not before biting into it with large-capacity biopsy forceps.
Another item in the guideline was the suggestion that an endoscopist can go right to an endoscopic mucosal resection if, for example, they found a small subepithelial lesion (≤1 cm) in the rectum that they suspected was a neuroendocrine tumor. This is a scenario that many endoscopists will encounter over the course of their career, and we wanted to give them the confidence to proceed and to know that they do not need an endoscopic ultrasound for this, and they don’t need to send the patient to someone else. It’s a very straightforward endoscopic mucosal resection that people can do when they first find the lesion, and that’s effectively a cure for that patient. The guideline document has a specific algorithm that includes the management of small rectal neuroendocrine tumors.
GEN: How might the new guideline change practice?
Dr. Jacobson: We’re hopeful that more endoscopists who don’t do endoscopic ultrasound will feel confident in some settings with referring a patient directly for surgery, bypassing the need for other invasive tests, such as an endoscopic ultrasound, or that they would be confident to, let’s say, remove a small rectal neuroendocrine tumor on their own. We are trying to be very patient-centered and efficient with our use of healthcare resources.
In addition, we recommend that endoscopists think very carefully before doing a bite-on-bite biopsy, to make sure that they’re really confident that there’s not a vessel in the wall that they’re mistaking as a solid mass. And for those who do more advanced endoscopic procedures and actually resect these lesions, the guideline will give people a sense of when endoscopic submucosal dissection is helpful or perhaps superior to a standard endoscopic mucosal resection.
GEN: What were some of the hottest points of debate among the guideline panelists, and how did you resolve them?
Dr. Jacobson: There really were no major areas of contention. We wrestled a little bit with the question of whether bite-on-bite is still a reasonable thing to do. Most of us had at least one story we could share about an endoscopist who thought something was solid and took a biopsy, only to find it was a vessel that then bled. We don’t like to base anything on anecdotes, but what GRADE does is help you focus and try to balance whether there are actual risks and not just benefits if you proceed in one direction versus another. So, we resolved the wording in that recommendation based on the concept of avoiding harm. At this point, the literature is not precise enough to allow a specific patient-focused recommendation that is strong.
GEN: Where are the remaining gaps in evidence?
Dr. Jacobson: One area where the literature is sparse relates to GI stromal tumors, which often are in the stomach. There wasn’t enough evidence to say you had to resect these when they are small. That means you’re going to need to do surveillance over time. There really was no definitive literature, so you end up with a best guess by a group of experts to say, maybe it should be every year or every two years. We’re very careful in those settings to say, we don’t know. The clinician should take the patient’s interests and their overall health into account when determining how often the patient should have an endoscopy, considering whether there are other reasons to do endoscopy more frequently or to monitor the patient for some other reason.
In addition, there are big gaps in the literature on the therapeutic side. As technologies are developing—with new tools that help with endoscopic submucosal dissection, suturing and closing wall defects, etc.—that’s really the area where researchers should be focusing. We would have loved to see more rigorous head-to-head, randomized controlled trials comparing some of these techniques. Data from such trials will be very helpful in the future to help guide clinicians.
One issue is the infrequency with which any one endoscopist finds such lesions. A big referral center still might not have enough cases to do a single-center study that’s of a reasonable size, so it likely will require organizing a large number of academic centers to have enough combined referral volume. It’s difficult, but there is a very real appetite to compare techniques. I have no doubt my colleagues are very, very creative, so I suspect we will see more robust data in the coming years.
Dr. Jacobson reported no relevant financial disclosures.
{RELATED-HORIZONTAL}