A recently developed scoring system could be a useful tool to noninvasively identify people with type 2 diabetes who are at risk for developing nonalcoholic steatohepatitis, researchers have found.
With the growing prevalence of nonalcoholic fatty liver disease in the general population, clinicians are eager to find ways to effectively screen patients for worsening liver fibrosis. In April 2020, researchers published a novel measurement, called the FibroScan-AST (FAST) score, as a way to noninvasively stratify patients at higher risk for NASH who may benefit from pharmaceutical interventions and clinical trials (Lancet Gastroenterol Hepatol 2020;5[4]:362-373). Although this measurement was validated in multiple populations, whether it could effectively screen a group of patients already at a higher risk for liver disease was unclear.
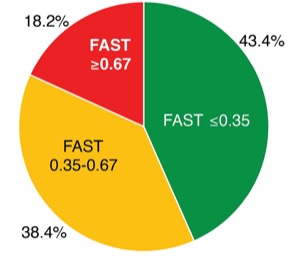
In a study presented at the 2020 virtual meeting of the American College of Gastroenterology (abstract S1190), researchers reported that in patients with type 2 diabetes, the FAST score classified 18% as having a high risk for NASH, and ruled out NASH in 43% of patients.
Liver biopsies remain the “gold standard” to identify NASH, but if FibroScan (Echosens) is available, the FAST score is a “simple way to risk-stratify patients with diabetes into low risk and high risk for fatty liver [disease],” said Naim Alkhouri, MD, the senior author of the new study and director of the fatty liver program at Arizona Liver Health, in Chandler.
The FAST score, a number between 0 and 1, uses the liver stiffness measurement and controlled attenuation parameter, both taken by FibroScan, combined with levels of aspartate aminotransferase to estimate a patient’s risk for NASH. A score at or above 0.67 indicates a high risk for NASH, while a score below 0.35 indicates that NASH is unlikely.
In a retrospective chart review, researchers calculated the FAST scores of patients with type 2 diabetes who underwent FibroScan at the Texas Liver Institute in San Antonio between January 2018 and January 2020. Of the 198 patients selected for the study, 107 (71.8%) were women and 79 (69.8%) were Hispanic. The score identified 36 patients (18.2%) at high risk for NASH, and 86 patients (43.4%) were classified as low risk. The remaining 76 (38.4%) fell in the indeterminate range, which indicates a need for further testing. Such tests could include MRI-based studies like magnetic resonance elastography or proton density fat fraction, or a liver biopsy, Dr. Alkhouri said.
That the study identified 18% of the population as high risk is “intrinsically valuable,” Elliot Tapper, MD, an assistant professor in the Division of Gastroenterology at the University of Michigan Medical Center, in Ann Arbor, told Gastroenterology & Endoscopy News. Now, clinical trial researchers can have a better idea of “how many people [they] have to screen to find a potential candidate,” Dr. Tapper said.
Federal regulations require a liver biopsy confirming fibrosis, and active steatohepatitis is necessary to enroll in clinical trials for NASH and NAFLD. However, if the results of noninvasive tests like FAST can motivate the stratified higher-risk patients to undergo liver biopsy, that can widen the candidate pool for clinical trials, Dr. Tapper added.
But the study does have a few important caveats, said Michelle Long, MD, MSc, an assistant professor at Boston University School of Medicine. Liver biopsies were not performed to determine whether patients were categorized correctly. Although the score was previously validated in multiple populations, she added, future studies should explore the utility of the FAST score in populations with a high prevalence of disease. Researchers also need to investigate whether high FAST scores correspond to liver-related outcomes to understand whether the FAST score can be a useful clinical tool, Dr. Long noted, saying, “there is more work that needs to be done.” But Dr. Alkhouri said he remains optimistic: “Once we have more data, I visualize this being a test that would be adequate on its own to qualify someone for treatment and then monitor their response to treatment.”
—Lucy Hicks