The PREEMPT CRC study, which evaluated a blood-based multiomics screening test for colorectal cancer, met its primary end points of sensitivity for CRC, specificity for advanced CRC neoplasia, and negative and positive predictive values for advanced CRC neoplasia, but with a lower magnitude of benefit than investigators had hoped.
“The sensitivity for CRC and advanced precursor lesions was lower than expected,” said Aasma Shaukat, MD, MPH, the Robert M. and Mary H. Glickman Professor of Medicine and Population Health and director of GI Outcomes Research at the NYU Grossman School of Medicine, in New York City, at a press briefing during the 2025 ASCO Gastrointestinal Cancers Symposium (abstract 18). But with continued optimization, she said, this blood test may provide a “convenient, effective option for colorectal cancer screening” for some patients.
The PREEMPT CRC study is the largest study of any blood-based CRC screening test, enrolling more than 40,000 average-risk adults 45 to 85 years of age at 200 sites across the United States. Participants’ blood samples were examined for molecular signals of advanced CRC cellular changes, with results matched to colonoscopy findings. The final population for sampling and colonoscopy consisted of 27,010 enrollees.
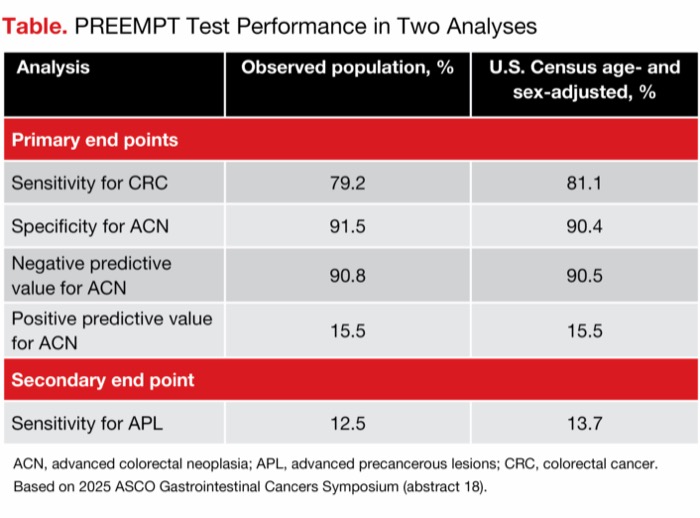
| Table. PREEMPT Test Performance in Two Analyses | ||
| Analysis | Observed population, % | U.S. Census age- and sex-adjusted, % |
|---|---|---|
| Primary end points | ||
| Sensitivity for CRC | 79.2 | 81.1 |
| Specificity for ACN | 91.5 | 90.4 |
| Negative predictive value for ACN | 90.8 | 90.5 |
| Positive predictive value for ACN | 15.5 | 15.5 |
| Secondary end point | ||
| Sensitivity for APL | 12.5 | 13.7 |
| ACN, advanced colorectal neoplasia; APL, advanced precancerous lesions; CRC, colorectal cancer. Based on 2025 ASCO Gastrointestinal Cancers Symposium (abstract 18). | ||
PREEMPT Performance Adjusted to Census Population
Because the demographic characteristics of the study participants did not exactly match the U.S. population for age and sex, the results were weighted to match U.S. Census data in a prespecified analysis. This analysis, which gives a “snapshot” of the test’s performance in a U.S. population, achieved results that were similar to the results of the unadjusted analysis, Dr. Shaukat noted (Table). “The results were robust in a prespecified direct standardization adjustment,” she said.
William M. Grady, MD, the Rodger Haggitt Professor of Medicine at the University of Washington School of Medicine and a professor in the Translational Science and Therapeutics Division of the Fred Hutchinson Cancer Center, in Seattle, put the findings into context with other available modalities. He noted that for detecting CRC, this test’s sensitivity is equivalent to the fecal immunochemical test (FIT) and is “slightly less” than the recently approved Shield (Guardant Health) blood-based test, but its specificity is “slightly higher.” For detecting advanced polyps, this test—like the Shield test—has a sensitivity of less than 14%, which is lower than that of FIT, multi-target stool (mt-sDNA) (Cologuard, Exact Sciences) and colonoscopy.
“Overall, the performance of this test is similar to [that of] the Shield test, which means that, if it becomes FDA approved, its appropriate use is for CRC screening of people who elect to not do colonoscopy, mt-sDNA, mt-sRNA or FIT testing,” Dr. Grady said. “It appears that a challenge for cell-free DNA-based screening tests at this time is the development of a test whose sensitivity is higher than FIT for advanced polyps, which would have a significant impact on its use in the clinic.”
—Caroline Helwick
Dr. Shaukat reported financial relationships with Freenome Holdings and Iterative. Dr. Grady reported financial relationships with DiaCarta, Freenome, GLG, Guardant, Guidepoint, Karius and LucidDx.
This article is from the May 2025 print issue.