Interest in modifying the gut flora by consuming foods or microbes that may improve overall health dates back to the early 1900s, when it was theorized that certain milks and yogurts may provide a health benefit to the populations consuming them.1
Just how probiotics may affect various conditions is not completely understood, but several mechanisms have been proposed. One is that the gut microbiome influences visceral hypersensitivity and pain and that Lactobacillus-induced expression of mu-opioid and cannabinoid receptors in the intestinal epithelium may be able to mediate pain in a manner similar to that of opioids.2 Another proposed mechanism is modulation of the immune system. Several studies have found that probiotics or their products suppress inflammatory cytokines and stimulate protective cytokines, mostly in models of inflammatory bowel disease (IBD).3-5 Finally, probiotics may promote integrity of the intestinal epithelium, protecting intestinal epithelial tight junctions and barrier function, and may create biofilms that secrete factors that can inhibit pathogen invasion.6,7
As understanding of the gut microbiota and complex interactions involved in inflammation, gut permeability, and dysbiosis advances, the potential of probiotics is appealing to clinicians and patients alike. However, while enthusiasm for probiotics has skyrocketed—particularly alongside other heavily promoted nutritional supplements—scant data support their use. Perhaps more importantly, the gastrointestinal diseases for which they have benefits and the species that confer these benefits remain unclear, resulting in confusion among clinicians and the general public.
This evidence-based review is an update of a 2018 review meant to guide the choice of a probiotic regimen where data appear strong and highlights areas where more research is needed before more conclusive recommendations can be made.
Evidence Search
The methods used for finding the recommendations involved searches via PubMed, OVID, and the Cochrane Review Library for the terms “probiotics,” “indications,” “dosing,” “pouchitis,” “infectious diarrhea,” “antibiotic-associated diarrhea,” “constipation,” “irritable bowel syndrome,” “hepatic encephalopathy,” “ulcerative colitis,” “Crohn’s disease,” and “Clostridioides.” Results were further narrowed into randomized controlled trials (RCTs), meta-analyses, and review articles in which information about specific strains and dosing could be found. Recommendations were selected based on the relative robustness of the relevant data.
Pouchitis
One of the best supported indications for the use of probiotics is pouchitis. In severe ulcerative colitis (UC) and familial adenomatous polyposis for which a total colectomy is required, the appropriate procedure is a proctocolectomy with ileal pouch–anal anastomosis. The most frequent long-term complication of this surgical correction is acute or chronic inflammation of the S- or J-shaped ileal pouch.8 Symptoms of pouchitis include abdominal pain, fever, hematochezia, urgency, and increased stool frequency. Patients with pouchitis have distinct microbial patterns that likely are due to fecal stasis and colonic metaplasia from the original ileal mucosa, creating an inflammatory milieu associated with bacterial species such as Bacteroidaceae and Clostridiaceae species.8 Enterococcaceae may have a role in maintaining immunologic homeostasis in the mucosa.9 The use of probiotics, particularly VSL#3 (Alfasigma)—a probiotic containing Bifidobacterium breve, B. longum, B. infantis, Lactobacillus acidophilus, L. plantarum, L. paracasei, L. bulgaricus, and Streptococcus thermophilus—has been found to be most effective in randomized, placebo-controlled trials in both primary and secondary prophylaxis.10,11 This probiotic appears to work by decreasing tumor necrosis factor (TNF)-alpha, interferon-gamma, and matrix metalloproteinases 2 and 9.
Primary prophylaxis has been studied at a dosage of 3 g per day for 12 months.12 Secondary prophylaxis of relapsing pouchitis has been studied at a dosage of 6 g per day from 9 months to 1 year.10 Although antibiotics remain the drug of choice for periods of inflammation, high-dose VSL#3 at a dosage of 2 sachets twice daily (3,600 billion bacteria/day) for 4 weeks has been found to be effective for the treatment of mild pouchitis (between 7 and 12 on the Pouchitis Disease Activity Index).13 The American Gastroenterological Association (AGA) probiotic guidelines note that if the feasibility and cost of this combination of bacterial strains are problematic, one may reasonably not prescribe probiotics.14
Infectious Diarrhea
Infectious Gastroenteritis
The role of probiotics in treating acute infectious diarrhea in adults and children recently has recently been scrutinized. The AGA released guidelines recommending against the use of probiotics in children with acute infectious gastroenteritis since most studies were performed outside the United States and, therefore, differences in host genetics, diet, sanitation, and endemic enteropathogens preclude generalization of the outcomes to the United States.14 Probiotic use in adults with acute infectious gastroenteritis is unproven. A 2010 meta-analysis that included 63 RCTs—using different probiotic preparations, but most commonly Lactobacillus GG and Saccharomyces boulardii—in adults and children found that probiotics reduced the overall risk for diarrhea lasting 4 or more days by 59% (relative risk, 0.41; 95% CI, 0.32-0.53) and the mean duration of diarrhea by 25 hours (95% CI, 16-34 hours).15
Travelers’ Diarrhea
The use of probiotics also has been studied for the prevention of travelers’ diarrhea. Specifically, Lactobacillus GG has been shown to be effective because it is resistant to acid and bile, adheres to ileal cells, and produces an antimicrobial substance. In a double-blind, placebo-controlled study from Finland, Lactobacillus GG was used to prevent travelers’ diarrhea, with protection rates of 1.8% to 39.5%.16 Another study found that taking Lactobacillus GG capsules at a dose of 2×109 bacteria starting 2 days before departure and continuing throughout the trip reduced the risk for diarrhea from 7.4% to 3.9% per day.17
Of note, a 2018 report in the Journal of Travel Medicine found that the data for probiotics were too weak to recommend use to prevent travelers’ diarrhea but that there was promising evidence for the prebiotic bifidobacterial-galacto-oligosaccharides (B-GOS).18In trials, B-GOS strongly inhibited the attachment and colonization of the gut pathogens Escherichia coli and Salmonella typhi. A double-blind, placebo-controlled study concluded that the group given the B-GOS mixture reported a statistically significant reduction in reported diarrhea compared with a placebo group (P<0.05). Combining prebiotics and probiotics into a single formulation (synbiotic) did not reduce the risk for developing travelers’ diarrhea. We do not believe there is sufficient evidence yet to warrant prophylactic probiotics for travelers’ diarrhea.
Antibiotic-Associated Diarrhea
The most common form of infectious diarrhea related to the use of antibiotics is caused by Clostridioides difficile and is referred to as C. difficile–associated diarrhea (CDAD). Probiotics have a defined role in its prevention. The mechanism of action includes alteration of intestinal flora, antimicrobial activity, intestinal barrier protection, and immunomodulation. A systematic review of probiotic use to prevent C. difficile infection in hospitalized patients found that probiotics reduced the risk for CDAD by more than 50% when they were taken within 2 days of the first antibiotic dose, with no evident increase in adverse events (AEs).19
There are 4 recommended probiotic regimens for the prevention of C. difficile infection. In adults and children on antibiotic treatment, the recommended regimens for preventing C. difficile infection include S. boulardii; a 2-strain combination of L. acidophilus CL1285 and L. casei LBC80R; a 3-strain combination of L. acidophilus, L. delbrueckii subspecies bulgaricus, and Bifidobacterium bifidum; or the 4-strain combination of L. acidophilus, L. delbrueckii subspecies bulgaricus, B. bifidum, and Streptococcus salivarius subspecies thermophilus.14 The beneficial effect of probiotics was seen mainly in patients with a very high risk for developing C. difficile infection (>15% baseline risk).
There is, however, insufficient evidence to recommend probiotics for treatment of active CDAD.
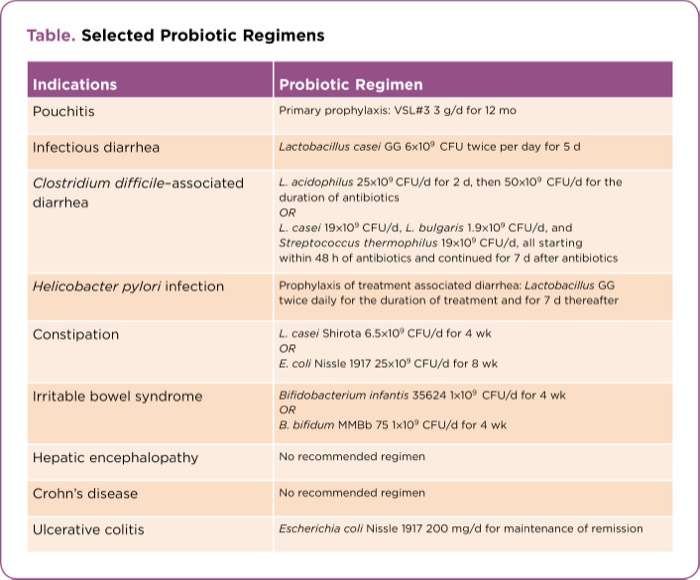
| Table. Selected Probiotic Regimens | |
| Indications | Probiotic Regimen |
|---|---|
| Pouchitis | Primary prophylaxis: VSL#3 3 g/d for 12 mo |
| Infectious diarrhea | Lactobacillus casei GG 6×109 CFU twice per day for 5 d |
| Clostridium difficile–associated diarrhea | L. acidophilus 25×109 CFU/d for 2 d, then 50×109 CFU/d for the duration of antibiotics OR L. casei 19×109 CFU/d, L. bulgaris 1.9×109 CFU/d, and Streptococcus thermophilus 19×109 CFU/d, all starting within 48 h of antibiotics and continued for 7 d after antibiotics |
| Helicobacter pylori infection | Prophylaxis of treatment associated diarrhea: Lactobacillus GG twice daily ?for the duration of treatment and for 7 d thereafter |
| Constipation | L. casei Shirota 6.5×109 CFU/d for 4 wk OR E. coli Nissle 1917 25×109 CFU/d for 8 wk |
| Irritable bowel syndrome | Bifidobacterium infantis 35624 1×109 CFU/d for 4 wk OR B. bifidum MMBb 75 1×109 CFU/d for 4 wk |
| Hepatic encephalopathy | No recommended regimen |
| Crohn’s disease | No recommended regimen |
| Ulcerative colitis | Escherichia coli Nissle 1917 200 mg/d for maintenance of remission |
Helicobacter pylori Infection
Another antibiotic-associated indication for probiotics is for patients undergoing therapy to eradicate Helicobacter pylori. In an updated, evidence-based international consensus, experts concluded that probiotics are helpful as adjuvant therapy to prevent or reduce the duration or intensity of AEs to H. pylori eradication therapy and improve compliance with treatment.20 In a randomized, placebo-controlled study, patients taking a standard H. pylori regimen supplemented with probiotics reported a lower incidence of AEs, including diarrhea, and overall treatment tolerability was improved.21 The probiotic was given during and for 7 days after H. pylori therapy; patients received one of several effective regimens, including Lactobacillus GG administered twice daily, S. boulardii given twice daily, or a combination of Lactobacillus and Bifidobacterium administered twice daily. A meta-analysis of 10 clinical trials of adjuvant probiotics in patients with H. pylori infection demonstrated increased cure rates with probiotic supplementation (pooled odds ratio [OR], 2.07; 95% CI, 1.40-3.06) and reduced the incidence of total antibiotic-related AEs (pooled OR, 0.31; 95% CI, 0.12-0.79).22
Constipation
Using probiotics in patients who have nonsevere chronic constipation and do not have irritable bowel syndrome (IBS) also has been studied, and the results remain unconvincing. A randomized, double-blind, placebo-controlled study showed that a probiotic beverage containing L. casei Shirota at a dosage of 6.5×109 colony-forming units (CFU) or 65 mL per day for 4 weeks resulted in a significant improvement in both stool frequency and consistency starting in the second week of intervention.23 In another RCT, E. coli Nissle 1917 at a dosage of 25×109 CFU for 8 weeks increased stool frequency.24 Although small studies have suggested an improvement in bowel movements, including frequency, stool consistency, and intestinal transit time with Bifidobacterium lactis DN-173 010, B. lactis BB12, L. casei Shirota, L. reuteri DSM 19738, and E. coli Nissle 1917, there was marked heterogeneity in study design and results, as well as publication bias.
Irritable Bowel Syndrome
Although data supporting probiotics for general IBS are limited largely by methodological shortcomings, there is some support for their use for the diarrhea variant (IBS-D). Controlled trials have shown that B. infantis 35624 at a dosage of 1×108 CFU per day for 4 weeks can improve abdominal pain, bloating, bowel dysfunction, incomplete evacuation, straining, and the passage of gas.25 One capsule of B. bifidum MIMBb75 dosed at 1×109 CFU over 4 weeks effectively alleviates global IBS and improves IBS symptoms simultaneously, with a subjectively reported improvement in quality of life.26 Studies examining a number of Lactobacillus species, including L. salivarius UCC4331, L. plantarum DSM9843, L. plantarum LPO1, and L. plantarum 299V, as well as B. bifidum MIMBb75, B. breve BR, and VSL#3, also have shown improvement in patient-reported symptoms, including flatulence and bloating, but have shown no overall effect on stool quality or frequency.
The ACG Monograph on Management of Irritable Bowel Syndrome, published in 2018, suggested probiotics can be taken to improve global symptoms as well as bloating and flatulence in patients with IBS.27 They reviewed 53 RCTs involving 5,545 patients and found that probiotics were statistically superior to placebo, with a number needed to treat of 71. In general, probiotics appeared to have beneficial effects on global IBS symptom scores or abdominal pain scores, bloating scores, and flatulence scores. In all 36 studies reviewed, involving 4,183 patients, there was no increased risk for AEs.
A more targeted microbiome approach is evolving. A systematic review of gut microbiota in IBS has described a decrease in the genus Bifidobacterium.28 To address this specific dysbiosis seen in IBS, a randomized, double-blind, placebo-controlled study explored human milk oligosaccharides (HMO), specifically a 4:1 mix of 2’-O-fucosyllactose and lacto-N-neotetraose and found that it increased the abundance of Bifidobacterium species in IBS patients.29 In an abstract, symptoms associated with moderate or severe IBS improved significantly at 12 weeks in adults who consumed a supplement containing HMO, with overall IBS severity score decreasing by 55%.30 This type of precision microbiome-tailored therapy will evolve in the coming years.
Hepatic Encephalopathy
In various forms of chronic liver disease, the liver—secondary to damage and diverted blood flow—loses the ability to clear ammonia from the body. The increasing levels of ammonia lead to a reversible encephalopathy known as hepatic encephalopathy. Treatment is aimed at either increasing the excretion of or decreasing the production of ammonia. Lactulose, a prebiotic, works via ion-trapping coupled with a potent laxative effect. Rifaximin (Xifaxan, Salix) works by decreasing the population of urease-producing bacteria. Probiotics are theorized to work by making the gut environment more favorable for non–urease-producing bacterial species or modifying the pH of the gut lumen, thereby decreasing the production of ammonia and preventing or reversing hepatic encephalopathy.31
An RCT has shown that a dose of 3 capsules per day containing 112.5 billion viable lyophilized bacteria per capsule, each containing 4 strains of Lactobacillus (L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subspecies bulgaricus), 3 strains of Bifidobacterium (B. longum, B. breve, and B. infantis), and 1 strain of Streptococcus salivarius (subspecies thermophilus), was more effective than no treatment for secondary prophylaxis of clinical hepatic encephalopathy, and was as effective as standard lactulose therapy.32 Overall, however, no mortality benefit has been identified to support the use of probiotics alone in the treatment of hepatic encephalopathy.33
Recently, the focus has shifted to analyzing the effect of fecal microbiota transplantation (FMT) in patients with hepatic encephalopathy.34 A study published in Hepatology found that FMT reduced hospitalizations and improved cognition and dysbiosis in cirrhosis with recurrent hepatic encephalopathy.35 In addition, FMT capsules were found to be safe and associated with improved duodenal mucosal diversity, dysbiosis, reduced lipopolysaccharide-binding protein, and improved EncephalApp performance. More research is needed to determine the best technique to target the microbiome to improve hepatic encephalopathy.
Crohn’s Disease and UC
Alterations in gut microbiota have been shown to play a role in the pathogenesis of Crohn’s disease (CD) and UC. As for many of the other indications discussed, strong evidence is lacking in IBD because of small sample sizes, variations in probiotic regimens, or variations in dosing. VSL#3, however, has shown some efficacy in inducing remission in mild to moderate UC. In an RCT (N=147), more patients with active mild to moderate UC taking VSL#3 (900 billion bacteria/day) had 50% improvement in the Ulcerative Colitis Disease Activity Index compared with those receiving placebo at 6 weeks (32.5% vs 10.0%; P=0.001).36 At week 12, 42.9% of patients taking VSL#3 achieved remission versus 15.7% of placebo-treated patients (P<0.001). A meta-analysis found that VSL#3, when added to conventional therapy at a dose of 3.6×1012 CFU/day, is safe and more effective than conventional therapy alone in achieving higher rates of response and remission in patients with mild to moderate UC.37
The literature also suggests that VSL#3 may reduce rates of relapse in quiescent UC. One RCT looked at the add-on use of VSL#3 (weight-based 450-1,800 billion bacteria/day) to a 5-aminosalicylate in the maintenance of remission in a small pediatric cohort with mild to moderate UC (N=29).38 Fewer patients treated with VSL#3 relapsed within 1 year of follow-up (21.4% vs 73.3%). At 6 months, 12 months, and time of relapse, endoscopic and histologic scores were lower in the VSL#3 group. In addition, in 2 RCTs, E. coli Nissle 1917 given at a dose of 200 mg per day (2.5-25×109 viable bacteria per capsule) for 12 months was found to be at least as effective as mesalamine in the prevention of relapse of UC during symptom-free periods in patients followed over 1 year.31,39,40 Furthermore, there were no significant differences between mesalamine and E. coli Nissle 1917 in safety and tolerability measures in UC patients. The AGA recommends the use of probiotics in UC only in the context of a clinical trial due to the lack of large RCTs and the cost of VSL#3.14
In CD, the data, again, are minimal and show either no significant difference between any studied probiotic strain alone and placebo or, less optimistically, show worse outcomes compared with standard medical therapies. In a small RCT, a dose of 2×1011 CFU of freeze-dried viable B. longum in a gelatin capsule, and a sachet containing 6 g of Synergy I (Orafti), given twice daily for 6 months, resulted in improvement in endoscopic and histologic scoring of CD compared with placebo alone.41 This intervention, however, did not lead to improvement in patient-reported symptoms. At the time of this writing, there were no data recommending the use of probiotics alone in the maintenance or induction of remission in CD, and the AGA recommends only to use probiotics in CD in the context of a clinical trial.14,42,43
Another approach to modulating the microbiome in CD is diet-based therapy. A multinational head-to-head RCT looked at a whole-food oral diet with partial enteral nutrition (EN) compared with standard-of-care exclusive EN in pediatric patients with mild to moderate CD.44 Both diets were effective in inducing remission by week 6, and the combination CD exclusion diet plus partial EN induced sustained remission in a significantly higher proportion of patients than exclusive EN; the combination also produced changes in the fecal microbiome associated with remission (decreases in Haemophilus, Veillonella, Anaerostipes, and Prevotella, and increases in Roseburia and Oscillibacter).
Preterm Low–Birth-Weight Babies
One of the most compelling indications for probiotics is to prevent necrotizing enterocolitis (NEC) in at-risk preterm (<37 weeks’ gestational age) low–birth-weight infants.14,21 NEC, an emergent condition associated with intestinal necrosis, can lead to short bowel syndrome and impaired neurodevelopment. The microbiome composition is different in infants with NEC compared with healthy infants, providing a possible therapeutic intervention with probiotics. The recommended combinations include Lactobacillus species and Bifidobacterium species (L. rhamnosus ATCC 53103 and B. longum subspecies infantis; L. casei and B. breve; L. rhamnosus, L. acidophilus, L. casei, B. longum subspecies infantis, B. bifidum, and B. longum subspecies longum; L. acidophilus and B. longum subspecies infantis; L. acidophilus and B. bifidum; L. rhamnosus ATCC 53103 and B. longum Reuter ATCC BAA-999; L. acidophilus, B. bifidum, B. animalis subspecies lactis, and B. longum subspecies longum; B. animalis subspecies lactis [including DSM 15954]; L. reuteri [DSM 17938 or ATCC 55730]; or L. rhamnosus [ATCC 53103 or ATC A07FA, or LCR 35]). These regimens were found to reduce all-cause mortality compared with placebo.
Cautions and Considerations
It is imperative that clinicians consider and discuss contraindications and risks when recommending and prescribing probiotics for patients. Hypothetically, probiotics may translocate, causing bacteremia or fungemia, as well as contamination of the product, and caution is advised when they are used in immunocompromised, hospitalized, or postoperative patients.45,46 In addition, commercially available probiotics may be contaminated with allergens, such as cow’s milk protein, and should be avoided in patients with severe allergies.47 An abstract from the Celiac Disease Center presented at the 2018 Digestive Disease Week found that there is significant contamination of probiotics with gluten; celiac patients should be cautious when using these products.48 In addition, there are not enough safety data on the use of probiotics during pregnancy, although published studies thus far have not found any AEs.49 Pregnant women also should avoid any unpasteurized products.
Another important consideration when counseling patients about probiotics is the utility of eating yogurt as a source of probiotics. Some yogurts made in the United States are pasteurized, a process that kills live bacterial cultures. In addition, studies have shown that live cultures in yogurt may not survive in the low pH of the product, they may not persist during prolonged shelf time or transit through the acidic stomach, and they may not resist degradation in the small intestine by hydrolytic enzymes and bile salts.50 It is not known how much of the live cultures reach the distal gut and colonize the microbiota after the aforementioned processes, leading to questions about the clinical utility of ingesting yogurt for the probiotic content. Other foods to consider that contain live cultures include kimchi (a Korean fermented cabbage dish), sauerkraut (fermented cabbage), miso (a fermented soybean-based paste), pickles, kombucha (a fermented tea), and apple cider vinegar (made from fermented apple sugars).
Conclusion
The data remain strongest for the use of probiotics in pouchitis at a dosage of 3 g per day of VSL#3 for primary prophylaxis, and 6 g per day for secondary prophylaxis. There is not enough evidence to use probiotics to treat C. difficile infection, but there are probiotic regimens that are effective as a preventive measure in patients while they are receiving antibiotic therapy. The evidence for microbiome-manipulating therapy in IBS and hepatic encephalopathy is promising. In IBD, probiotics can be considered for the induction of remission and maintenance in mild to moderate UC.
Although the proposed health benefits of probiotics continue to trend on social media and remain an area of active clinical research, it is clear that there is a shift toward other modalities of microbiome-based therapy. With confounding variables such as heterogeneity in study design, many researchers have started focusing on the other ways to induce changes in the microbiome, including diet, prebiotics, and FMT. In the near future, microbiome therapy will become more targeted and individualized, and precision microbiome manipulation will become a reality.
Patients will continue to ask about probiotics. When discussing probiotic use with patients, ensure to review the risks, benefits, and contraindications of their use. Consider concentrated, rigorously tested probiotic strains, in addition to foods that are able to deliver live bacterial cultures past the initial stages of digestion. Ensure patients are taking the correct regimen for the appropriate indication, and caution them that the data for other diseases are preliminary and likely will advance in the coming years. And stay tuned—the recommendations on probiotic strains and dosing will change as research continues in this rapidly evolving field.
References
- Brown AC, Valiere A. Probiotics and medical nutrition therapy. Nutr Clin Care. 2004;7(2):56-68.
- Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35-37.
- Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105(43):16731-16736.
- McCarthy J, O’Mahony L, O’Callaghan L, et al. Double blind, placebo-controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52(7):975-980.
- Lin YP, Thibodeaux CH, Pena JA, et al. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis. 2008;14(8):1068-1083.
- Seth A, Yan F, Polk DB, et al. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G1060-G1069.
- Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009;9:35.
- Shen B. Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol. 2013;11(12):1538-1549.
- Scarpa M, Grillo A, Faggian D, et al. Relationship between mucosa-associated microbiota and inflammatory parameters in the ileal pouch after restorative proctocolectomy for ulcerative colitis. Surgery. 2011;150(1):56-67.
- Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(2):305-309.
- Komanduri S, Gillevet PM, Sikaroodi M, et al. Dysbiosis in pouchitis: evidence of unique microfloral patterns in pouch inflammation. Clin Gastroenterol Hepatol. 2007;5(3):352-360.
- Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124(5):1202-1209.
- Gionchetti P, Rizzello F, Morselli C, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007;50(12):2075-2082; discussion 2082-2084.
- Su GL, Ko CW, Bercik P, et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020;159:697-705.
- Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
- Oksanen PJ, Salminen S, Saxelin M, et al. Prevention of travellers’ diarrhoea by Lactobacillus GG. Ann Med. 1990;22(1):53-56.
- Hilton E, Kolakowski P, Singer C, et al. Efficacy of Lactobacillus GG as a diarrheal preventive in travelers. J Travel Med. 1997;4(1):41-43.
- Evans DP. Non-pharmacotherapeutic interventions in travellers diarrhoea (TD). J Travel Med. 2019;25(suppl 1):S38-S45.
- Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
- Cremonini F, Di Caro S, Covino M, et al. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97(11):2744-2749.
- Koebnick C, Wagner I, Leitzmann P, et al. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol. 2003;17(11):655-659.
- Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47(1):25-32.
- Tilley L, Keppens K, Kushiro A, et al. A probiotic fermented milk drink containing Lactobacillus casei strain Shirota improves stool consistency of subjects with hard stools. Int J Probiotics Prebiotics. 2014;9(1-2):23-29.
- Mollenbrink M, Bruckschen E. [Treatment of chronic constipation with physiologic Escherichia coli bacteria. Results of a clinical study of the effectiveness and tolerance of microbiological therapy with the E. coli Nissle 1917 strain (Mutaflor)] [In German]. Med Klin (Munich). 1994;89(11):587-593.
- Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581-1590.
- Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(10):1123-1132.
- Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2018;113(suppl 2):1-18.
- Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108.
- Iribarren C, Törnblom H, Aziz I, et al. Human milk oligosaccharide supplementation in irritable bowel syndrome patients: a parallel, randomized, double-blind, placebo-controlled study. Neurogastroenterol Motil. 2020;32(10):e13920.
- Palsson OS, Peery A, Seitzberg D, et al. Human milk oligosaccharides improve all the central symptoms of irritable bowel syndrome: a multicenter, open-label trial. Am J Gastroenterol. 2019;114(suppl):abstract 467.
- Dalal R, McGee RG, Riordan SM, et al. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716.
- Agrawal A, Sharma BC, Sharma P, et al. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012;107(7):1043-1050.
- Khungar V, Poordad F. Hepatic encephalopathy. Clin Liver Dis. 2012;16(2):301-320.
- Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66(6):1727-1738.
- Bajaj JS, Salzman NH, Acharya C, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology. 2019;70(5):1690-1703.
- Sood A, Midha V, Makharia GK, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7(11):1202-1209, 1209.e1.
- Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20(9):1562-1567.
- Miele E, Pascarella F, Giannetti E, et al. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104(2):437-443.
- Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53(11):1617-1623.
- Rembacken BJ, Snelling AM, Hawkey PM, et al. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354(9179):635-639.
- Steed H, Macfarlane GT, Blackett KL, et al. Clinical trial: the microbiological and immunological effects of synbiotic consumption – a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol Ther. 2010;32(7):872-883.
- Lichtenstein L, Avni-Biron I, Ben-Bassat O. Probiotics and prebiotics in Crohn’s disease therapies. Best Pract Res Clin Gastroenterol. 2016;30(1):81-88.
- Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
- Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition tnduces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157(2):440-450.e8.
- Borriello SP, Hammes WP, Holzapfel W, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003;36(6):775-780.
- Liong MT. Safety of probiotics: translocation and infection. Nutr Rev. 2008;66(4):192-202.
- Bruni FM, Piacentini GL, Peroni DG, et al. Cow’s milk allergic children can present sensitisation to probiotics. Acta Paediatr. 2009;98(2):321-323.
- Nazareth S, Lebwohl B, Voyksner JS, et al. Widespread contamination of probiotics with gluten, detected by liquid chromatography-mass spectrometry. Gastroenterology. 2015;148(4):S-28.
- Dugoua JJ, Machado M, Zhu X, et al. Probiotic safety in pregnancy: a systematic review and meta-analysis of randomized controlled trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. J Obstet Gynaecol Can. 2009;31(6):542-552.
- Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000;78(1):80-88.
Editor’s note: Readers should be aware that the probiotic VSL#3 described in the referenced studies is the original formulation (the “De Simone Formulation”) that currently is being sold exclusively as Visbiome by ExeGi.
Copyright © 2021 McMahon Publishing, 545 West 45th Street, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form.

Download to read this article in PDF document:![]() Probiotics: A Review for Clinical Use
Probiotics: A Review for Clinical Use