In part 1 of the DDW Expert Picks column, Drew Schembre, MD, the director of the Digestive Health Care at John Muir Health, in Walnut Creek, Calif., reviews the top abstracts from the ASGE program and Monika Sarkar, MD, MAS, the director of the Women’s Liver Program at University of California, San Francisco, reviews her top hepatology-related abstracts from the AASLD program. Look for part 2, which will feature the top abstracts from the AGA and SSAT programs at DDW.
Cloud-based artificial intelligence for detection of colorectal neoplasia–a randomized controlled trial (EAGLE trial). Abstract 366.
A multicenter team of researchers in Europe conducted a randomized controlled trial to compare polyp detection using a cloud-based artificial intelligence computer-aided detection (CADe) system versus standard colonoscopy. The CADe system analyzes endoscopy images in real time and highlights suspected polyps with bounding boxes.
The training data used in this study included a high proportion (~20%) of sessile serrated lesions (SSLs) and large polyps, which was hypothesized to increase their detection during CADe use in the clinic, overcoming a shortcoming of other CADe systems seen in previous trials.
The colonoscopies were performed by endoscopists (n=22) from eight centers across four countries in Europe who each had conducted more than 1,000 colonoscopies and had an adenoma detection rate (ADR) of at least 25%. Participating patients (n=841) were undergoing screening or surveillance colonoscopies and were at least 40 years of age and at average risk for colorectal cancer. Patients were randomized 1:1 to the cloud-based CADe system (CADDIE, Odin Vision) or standard colonoscopy. The primary end points were superiority of the AI system for adenomas per colonoscopy (APC) and noninferiority of the system for the percentage of resected polyps that were confirmed adenomas (positive percent agreement [PPA]).
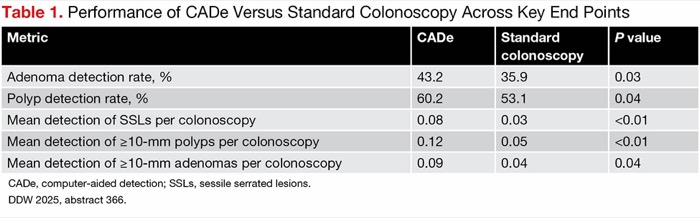
Both co-primary end points were met. The APC in the CADe arm was 0.82, compared with 0.62 in the standard colonoscopy arm (risk ratio, 1.33; 95% CI, 1.06-1.67). The PPA in the CADe arm was 53.9%, compared with 53.4% in the standard colonoscopy arm (difference, 0.5%; 95% CI, –5.0% to infinity). Additional metrics, including those for clinically significant polyps (e.g., SSLs, large polyps), were also improved in the CADe arm (Table 1).
| Table 1. Performance of CADe Versus Standard Colonoscopy Across Key End Points | |||
| Metric | CADe | Standard colonoscopy | P value |
|---|---|---|---|
| Adenoma detection rate, % | 43.2 | 35.9 | 0.03 |
| Polyp detection rate, % | 60.2 | 53.1 | 0.04 |
| Mean detection of SSLs per colonoscopy | 0.08 | 0.03 | <0.01 |
| Mean detection of =10-mm polyps per colonoscopy | 0.12 | 0.05 | <0.01 |
| Mean detection of =10-mm adenomas per colonoscopy | 0.09 | 0.04 | 0.04 |
| CADe, computer-aided detection; SSLs, sessile serrated lesions. DDW 2025, abstract 366. | |||
Dr. Schembre: A lot has been written about CADe over the last half decade. Studies have shown small but significant improvements in ADRs; however, most of the polyps identified only by CADe were small and often of questionable clinical significance. This large multicenter, randomized study differs in that it uses a cloud-based system with very rapid processing (99.7% of lesions identified in <100 mseconds) trained on newer data sets with an abundance of large, flat polyps. This appears to translate into improved detection of both large flat adenomas and sessile serrated adenomas—lesions that, if missed, could progress to cancer before the next colonoscopy. In addition, the platform purports to be updatable in real time, suggesting that with larger data sets and training, polyp detection could continue to improve. However, it does not address important issues such as user fatigue, procedure time and assessment of how much of the colon the endoscopist (and computer) actually view.
Multicenter, randomized non-inferiority trial comparing transanal minimal invasive surgery (TAMIS) and endoscopic submucosal dissection (ESD) for resection of non-pedunculated rectal lesions. Abstract 857.
Researchers in the Netherlands compared the efficacy, safety, patient burden and costs associated with TAMIS and ESD for resection of large nonpedunculated rectal lesions.

Patients with lesions greater than 2 cm and no more than 15 cm from the anal verge were randomized 1:1, stratified by polyp size (20-40 vs. >40 mm) and distance from the dentate line (<10 vs. 10-99 vs. 100-150 mm), to TAMIS or ESD. Patients were not eligible if they had deep submucosal invasion.
Data were analyzed based on intention to treat. The primary end point was noninferiority (margin, 6%) of cumulative local recurrence at 12 months. The secondary end points were radical resection rate, complication rate, quality of life, functional outcomes and cost-effectiveness.
A total of 198 patients were included in the study (100 ESD, 98 TAMIS). The mean age was approximately 67 years and 60% were male. About 60% of polyps were less than 40 mm, and the majority (56%) were 10 to 99 mm from the dentate line. During the study, one patient crossed over from ESD to TAMIS, and five crossed over from TAMIS to ESD.
Overall, ESD procedures were longer than TAMIS procedures (108 vs. 80 minutes; P<0.001), but not after adjusting for lesion size (11.1 vs. 10.7 minutes per cm2). TAMIS was associated with longer post-procedural hospital stay (2.23 vs. 1.77 days; P=0.023).
By 12 months, no patients in the ESD group and six in the TAMIS group had experienced recurrence (difference, –6.4%; 95% CI, –11.3% to –1.4%). The radical resection rate (R0) was higher in the ESD group (83% vs. 71%; P=0.002) overall, but there was no difference for invasive lesions (T1).
Patient-reported ColoRectal Functional Outcome score was comparable overall, but those in the TAMIS group had higher incontinence subscale scores from four days to six months post-procedure.
The investigators found that the initial procedure cost of ESD was lower than that of TAMIS (2,628 vs. 3,365 euros), but the costs over the 12 months post-procedure were comparable (7,135 vs. 7,216 euros).
Dr. Schembre: Historically, endoscopic approaches to large rectal lesions have taken a back seat to TAMIS because of the perception that TAMIS provides a deeper, more complete resection. This is the first randomized trial of ESD versus TAMIS for large nonpedunculated rectal lesions, and it clearly demonstrates noninferiority of ESD in expert hands. Moreover, several outcomes, such as length of stay, percentage of R0 resections and local recurrence, favored ESD. Given that comfort with ESD has grown in the United States and rectal ESD often represents the entry point for advanced endoscopists learning the techniques, we should expect to see an increase in the proportion of lesions approached by ESD in the future. However, the real-world results, especially in the early period, probably won’t mirror those of this study.
Incidence of lymph node metastasis stratified by submucosal invasion depth in non-pedunculated pT1 colorectal cancer: results from a multi-center JSCCR study of 4254 cases. Abstract 369.
Investigators in Japan quantified the risk for lymph node (LN) metastases in patients with nonpedunculated pT1 CRC related to disease factors, including submucosal invasion depth, lymphovascular invasion, high-grade tumor budding (BD) and poorly differentiated histologic type (POR).
Patients across 27 sites who underwent endoscopic or surgical resection from 2009 to 2016 were included in the study. Patients were not eligible if they had synchronous CRC, inflammatory bowel disease or polyposis.
In total, 4,252 patients were included in the analyses. The median age was 68 years, 58.4% were male, and tumors were relatively evenly split across the right-sided (34.5%), left-sided (29.6%), and rectal (35.9%) locations, with a median size of 20 mm.
Over a median of 5.2 years of follow-up, 7.7% of patients developed LN metastases and 1.6% developed distant metastases. The incidence of LN metastases increased as submucosal depth increased (0-999 mcm, 1.2%; 1,000-1,999 mcm, 6.2%; 2,000-2,999 mcm, 9.5%; =3,000 mcm, 11.6%).
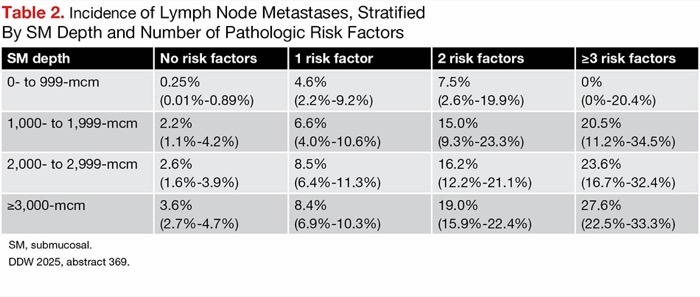
At a given submucosal depth, the incidence of LN metastases generally increased as the number of pathologic risk factors, including Ly1, V1, BD2/3, POR, mucinous carcinom, and signet-ring cell carcinoma, present increased (Table 2). To the investigators, these results “[underscore] the stronger predictive value of combined qualitative pathological risk factors over [submucosal depth] alone.”
| Table 2. Incidence of Lymph Node Metastases, Stratified By SM Depth and Number of Pathologic Risk Factors | ||||
| SM depth | No risk factors | 1 risk factor | 2 risk factors | =3 risk factors |
|---|---|---|---|---|
| 0- to 999-mcm | 0.25% (0.01%-0.89%) | 4.6% (2.2%-9.2%) | 7.5% (2.6%-19.9%) | 0% (0%-20.4%) |
| 1,000- to 1,999-mcm | 2.2% (1.1%-4.2%) | 6.6% (4.0%-10.6%) | 15.0% (9.3%-23.3%) | 20.5% (11.2%-34.5%) |
| 2,000- to 2,999-mcm | 2.6% (1.6%-3.9%) | 8.5% (6.4%-11.3%) | 16.2% (12.2%-21.1%) | 23.6% (16.7%-32.4%) |
| =3,000-mcm | 3.6% (2.7%-4.7%) | 8.4% (6.9%-10.3%) | 19.0% (15.9%-22.4%) | 27.6% (22.5%-33.3%) |
| SM, submucosal. DDW 2025, abstract 369. | ||||
Dr. Schembre: Discussion of risk factors for nodal and distant spread of T1 colon cancers traditionally has centered on depth of invasion, with general comfort with lesions invading less than 1,000 mcm but with growing anxiety as invasion reached 2,000 mcm or more. This very large, albeit retrospective, study demonstrates that while increased depth of invasion correlates with increased risk for spread, a variety of pathologic risk factors may influence nodal spread more. The presence of multiple risk factors greatly increases the risk for spread, even for relatively shallow lesions, while deep lesions with no risk factors appear to rarely spread. This matrix approach of depth of invasion along with pathologic risk factors should provide a more accurate risk assessment of nodal spread (and thus the relative indication for completion surgery), as an increasing number of T1 lesions are resected endoscopically.
Once-monthly efimosfermin alfa (BOS-580) in metabolic dysfunction-associated steatohepatitis with F2/F3 fibrosis: results from a 24 week, randomized, double-blind, placebo-controlled, Phase 2 trial. Abstract 723.
Investigators conducted a phase 2 trial of the FGF21 analog efimosfermin (Boston) to assess the drug’s safety, tolerability and efficacy in patients with metabolic dysfunction–associated steatohepatitis who had F2/F3 fibrosis.
For inclusion, these F2/F3 MASH patients were required to have a nonalcoholic fatty liver disease activity score (NAS) of at least 4, body mass index of at least 27 kg/m2, an MRI proton density fat fraction (PDFF) of at least 8% and an aspartate aminotransferase level greater than 25 U/L.
Participants were randomized 1:1 to either 300 mg of efimosfermin (n=43) or placebo (n=41) every four weeks for 24 weeks. At baseline, the mean age of participants was 54 years, 52% were female, mean BMI was 37.3 kg/m2, mean NAS was 5.1 and 43% had F3 fibrosis.
There were two serious adverse events (SAEs) seen in the efimosfermin arm, one of which was drug related (vs. zero SAEs in the placebo arm), and two adverse events led to discontinuation. Otherwise, the majority of treatment-emergent AEs were mild to moderate, most commonly nausea (33% efimosfermin vs. 13% placebo), diarrhea (23% efimosfermin vs. 8% placebo) and vomiting (16% efimosfermin vs. 3% placebo).
When assessing immunogenicity, the investigators found one patient in each arm had evidence of anti-efimosfermin antibodies and two patients in the efimosfermin arm had anti-FGF21 antibodies.
In their exploratory efficacy analyses, the investigators analyzed data for the 31 patients randomized to efimosfermin and 34 randomized to placebo who completed treatment and had two biopsies (one at baseline, one during weeks 20-28). A higher proportion of patients in the efimosfermin arm than in the placebo arm had fibrosis improvement without worsening of MASH (45% vs. 21%; P=0.038), MASH resolution without worsening of fibrosis (68% vs. 29%; P=0.002), and fibrosis improvement and MASH resolution (39% vs. 18%; P=0.066). These patterns also were seen in the subgroup with F3 fibrosis, although the differences did not reach statistical significance.
The exploratory efficacy analysis also showed that by week 24 the efimosfermin arm had improved PDFF reduction, greater reductions in liver injury biomarkers (alanine and aspartate aminotransferase) and a higher proportion of patients with normalized ALT and AST, greater improvements in triglycerides and high-density lipoprotein cholesterol, and in the subgroup with type 2 diabetes, hemoglobin A1c improvement.
Dr. Sarkar: This phase 2 study demonstrated improvement in markers and contributors to fibrosis progression, including ALT levels, severity of steatosis, lipids and HbA1c, while impressively resulting in fibrosis regression. The prolonged half-life of this formulation supports ease of use with once-monthly dosing. We will await phase 3 data on efimosfermin, which offers a promising future therapeutic option to add to our toolbox for the management of MASH fibrosis.
Initiation of secondary SBP prophylaxis in patients with a new SBP associates with a higher rate of non-SBP infections compared to those not started on it in a national cohort of veterans with cirrhosis. Abstract 6.
A multicenter team of investigators used data from the VA Corporate data warehouse to conduct a retrospective study of patients with cirrhosis who had spontaneous bacterial peritonitis (SBP) to understand the impact of secondary SBP prophylaxis (SecSBPPr) on SBP recurrence and incidence of non-SBP infections.
Patients with cirrhosis and SBP were identified for inclusion based on the presence of validated ICD codes between 2009 and 2019. SecSBPPr was defined as at least 120 days of continuous use of fluoroquinolones or trimethoprim-sulfamethoxazole (TMP-SMX), with at least two refills. The investigators assessed SBP recurrence and incidence of non-SBP infections, such as pneumonia, urinary tract infections (UTIs), bacteremia and Clostridioides difficile colitis from 30 days to two years post-SBP.
In total, 4,673 patients with cirrhosis and SBP survived their index SBP; 54.3% of this group began SecSBPPr (84.4% fluoroquinolones, 14.6% TMP-SMX). Those who started SecSBPPr were more likely to be white (81.1% vs. 77.7%) and to have slightly higher baseline Charlson Comorbidity Index (5.52 vs. 5.02), Model for End-Stage Liver Disease Sodium (MELD-Na) score (18.3 vs. 17.9) and albumin (2.81 vs. 2.72 g/dL) than those who did not start SecSBPPr.
A higher proportion of patients who started SecSBPPr experienced SBP recurrence (24.1% vs. 13.7%; P<0.001) and non-SBP infections (32.2% vs. 27.5%; P<0.001), particularly UTIs (14.9% vs. 11.9%; P=0.003). Those who did not start SecSBPPr were more likely to have no infection during the follow-up period (64.5% vs. 54.2%; P<0.001).
In their multivariate logistic regression model, adjusting for white race, Charlson Comorbidity Index, VA Center Complexity, MELD-Na, albumin, white blood cell count, proton pump inhibitor use, lactulose use, rifaximin use, propranolol use and nadolol use, the investigators found that use of SecSBPPr was associated with 1.21 times the odds of UTI (95% CI, 1.01-1.45) and 1.26 times the odds of any non-SBP infection (95% CI, 1.10-1.44).
Dr. Sarkar: Current guidelines support the use of SecSBPPr, although evidence supporting this practice is limited. Recently published data found SecSBPPr to be associated with a higher risk for recurrent SBP. The current study expands upon the same data set to demonstrate a higher adjusted risk for non-SBP infections with SecSBPPr. This finding was largely driven by incident UTIs, which shares overlapping bacteriology with SBP. Thus, the findings support the premise that SecSBPPr may promote microbial resistance and highlight the need for reevaluation of our current clinical practice.
A randomized controlled trial of hepatology specialty clinic-based stepped treatment to reduce unhealthy alcohol use in vulnerable patients with chronic liver disease. Abstract 1.
Researchers conducted a trial of a stepped alcohol treatment (SAT) model in patients with unhealthy alcohol use attending hepatology clinics at three healthcare systems between March 2022 and February 2024.
Unhealthy alcohol use was defined as more than seven drinks per week or four or more on a given day for women and more than 14 drinks per week or five or more on a given day for men. The SAT included three sessions of motivational interviewing, occurring every two weeks, followed by offer of addiction medicine and pharmacotherapy if alcohol use was still at unhealthy levels at month 3. Patients not randomized to SAT received usual care.
Among 157 enrolled patients, 81 were randomized to SAT and 76 to usual care. Patients had a median age of 61 years,14% were female, 17% had less than high school education and 54% had an annual income of $30,000 or less. Nearly half had cirrhosis (48%), 25% had current decompensation, 12% had prior decompensation and the median MELD-Na score was 9.
At three and six months, the mean drinks per week was comparable in the SAT and usual care arms. However, those in the SAT arm had a significantly larger decrease in drinks per week from baseline to three months (–1.25 vs. –0.78; difference, –0.47; 95% CI, –0.89 to –0.05) and baseline to six months (–1.69 vs. –1.14; difference, –0.48; 95% CI, –0.90 to –0.06), compared with those in the usual care arm. This trend was consistent upon multivariable analysis, adjusting for age, sex, race, ethnicity, cirrhosis status, an Alcohol Use Disorders Identification Test score of at least 8 (vs. <8), patient-reported importance and confidence to decrease alcohol use, and liver disease quality-of-life score.
At six months, participants had improvements in liver disease quality of life, anxiety scores and depression scores that were comparable across treatment groups.
The investigators found that the six-month odds of death or hospitalization were similar across treatment groups (odds ratio, 0.84; P=0.70).
Dr. Sarkar: With rising rates of alcohol-related liver disease (ALD), there is a growing need for therapeutic interventions. Stepped approaches have been shown to be beneficial for alcohol use disorder outside the context of liver disease, and the current study now evaluates the role of this intervention for ALD. The investigators found that SAT reduced the amount of alcohol intake from baseline to three- and six-month follow-up, independent of patient demographics, baseline alcohol consumption and presence of cirrhosis. Importantly, this study was conducted among vulnerable populations seen in county hospital or Veterans Affairs settings, supporting this intervention for those with otherwise constrained resources.
—Compiled and written by Natasha Albaneze, MPH
Dr. Sarkar reported financial relationships with Gilead, GSK and Novo Nordisk. Dr. Schembre reported financial relationships with Cook and Neptune.nonbreaking space
This article is from the July 2025 print issue.