The FDA granted approval for Amneal Pharmaceuticals Abbreviated New Drug Application for single-dose vials of propofol injectable emulsion USP, 200 mg/20 mL (10 mg/mL), 500 mg/50 mL (10 mg/mL) and 1,000 mg/100 mL (10 mg/mL).
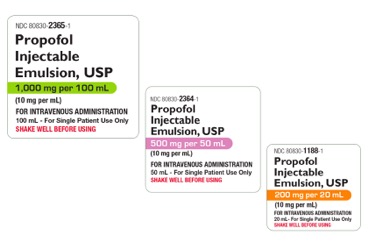
Propofol is an IV drug commonly used for the induction and maintenance of anesthesia and sedation that is currently on the ASHP drug shortages list due to supply chain constraints. Amneal said it will manufacture the product in-house on a dedicated line to provide consistent
